78812021-EN CardioMem®CM 100 XT 3
Contents
1Information about this Manual...............................................5
2Intended use..........................................................................6
3Indications and contraindications ..........................................7
3.1 Indications......................................................................7
3.2 Contraindications...........................................................7
4Regulatory information ..........................................................8
4.1 MDR compliance ...........................................................8
4.2 Radio frequency compliance.........................................8
4.3 Classifications................................................................8
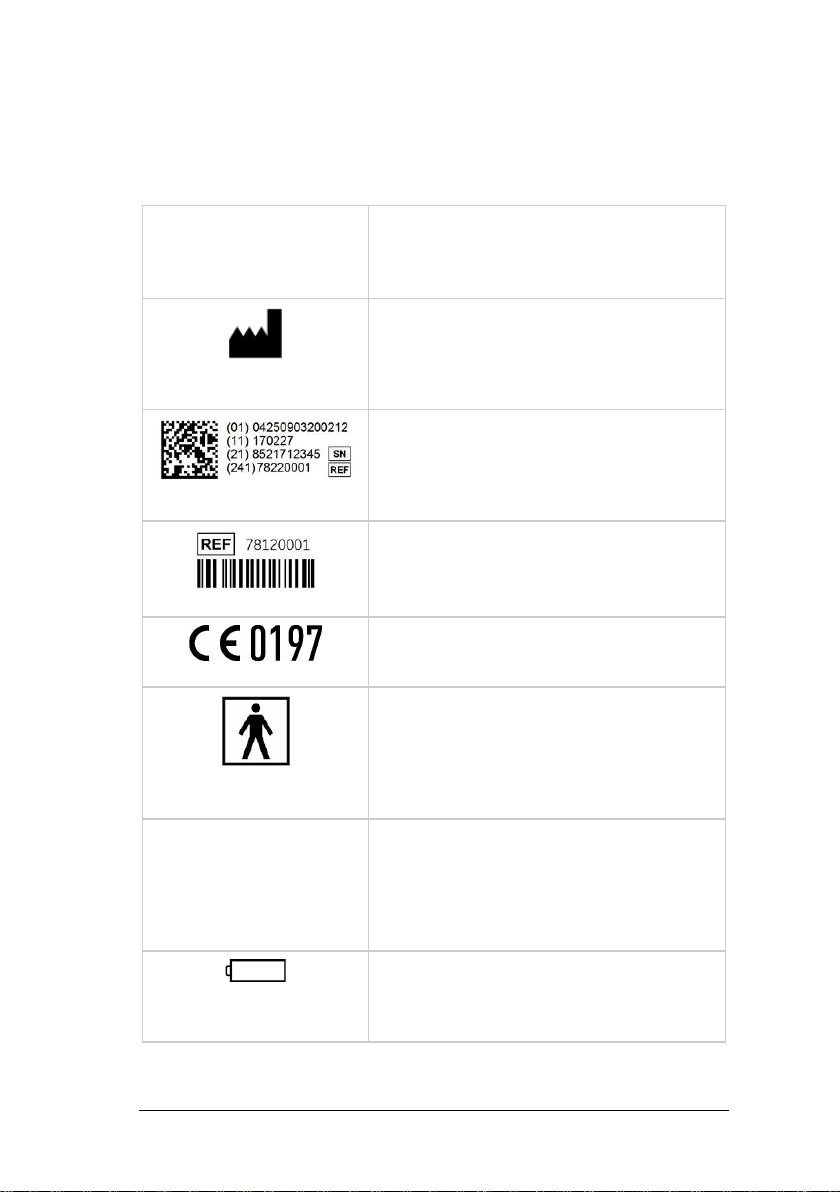
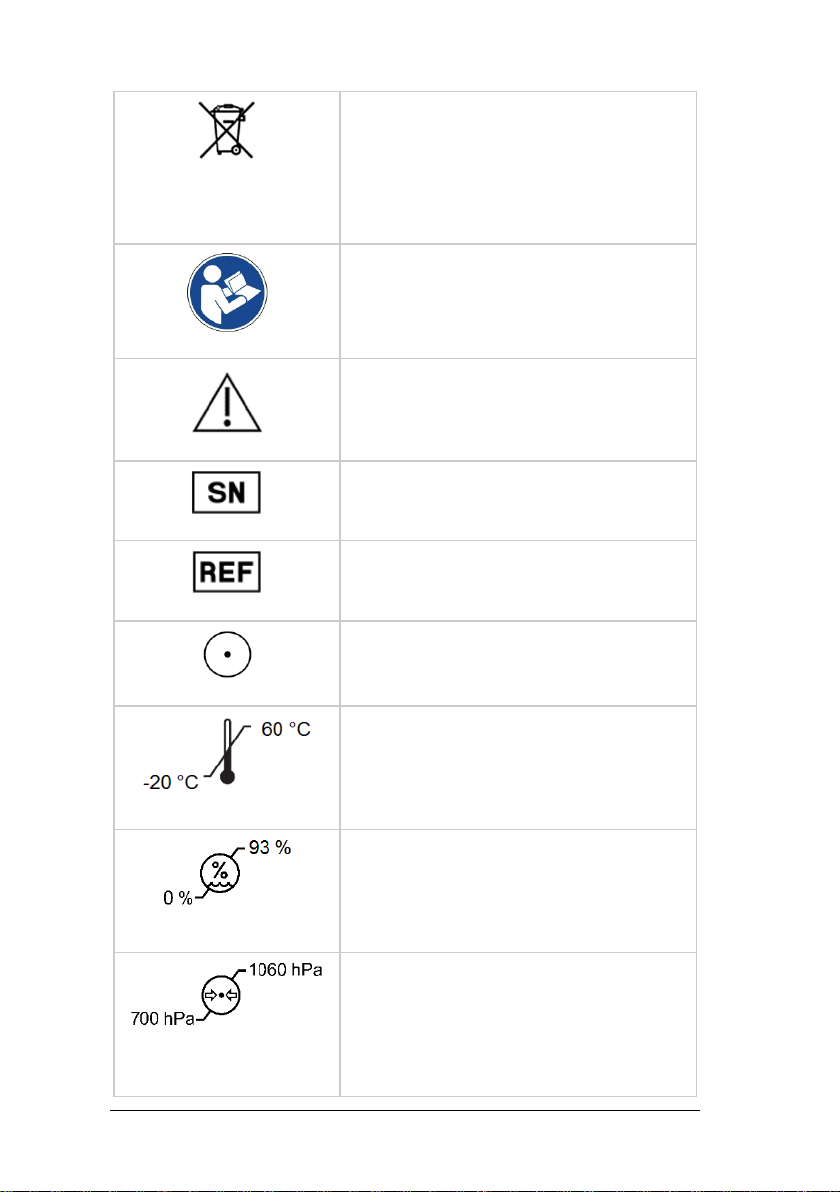
5Labelling ................................................................................9
6Safety information................................................................12
6.1 Definitions....................................................................12
6.2 General warning notes ................................................12
6.3 General precautions ....................................................15
6.4 Incident Reporting .......................................................19
7Warranty and service information........................................20
8Cleaning and disinfection ....................................................21
9Operating elements .............................................................22
9.1 Pushbutton...................................................................22
9.2 Visual and acoustic signals .........................................23
9.3 Lead scheme...............................................................24
10 Preparing the recording.......................................................25
10.1 Inserting the battery.....................................................25
10.2 Instructing the patient ..................................................27
10.2.1 Saving an event manually ...................................27
10.2.2 Recording diary....................................................28
10.2.3 Medical Emergency.............................................28
10.3 Preparing the skin of the patient..................................28
10.4 Connecting the electrodes to the recorder ..................29
10.5 Attaching the device to the patient ..............................30
10.6 Turning on the recorder...............................................31
10.7 Checking the signal quality..........................................31
11 Recording an event .............................................................32
11.1 Manual recording by pressing the pushbutton ............32
11.2 Automatic recording.....................................................32
11.2.1 Automatic detection of arrhythmias.....................32
11.2.2 Time-triggered recording .....................................33
12 End of recording ..................................................................34
12.1 Removing the electrodes.............................................34
13 Using the CM 100 Configurator software ............................35