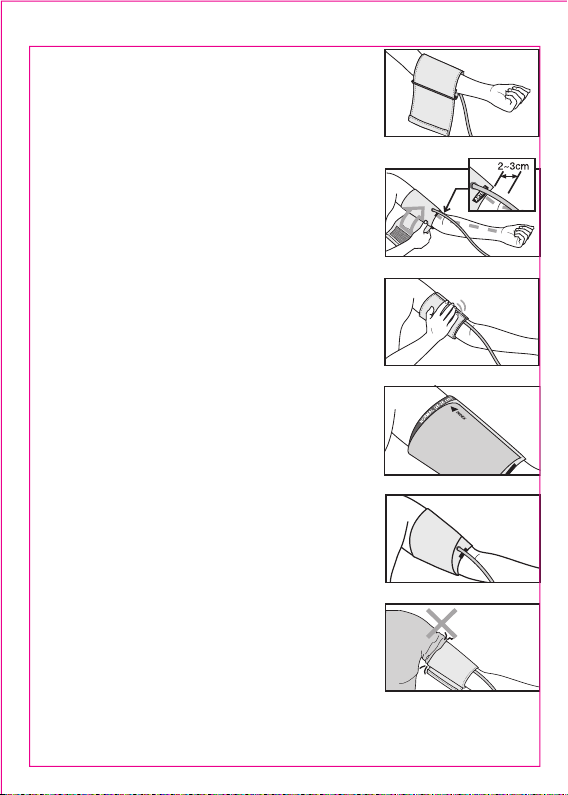
PREPARATION FOR MEASUREMENT
1. Do not conduct any measurements if the temperature is low (below 50°F /
10°C) or high (over 104°F / 40°C), or if the relative humidity is higher than
85%, as this can lead to inaccurate readings.
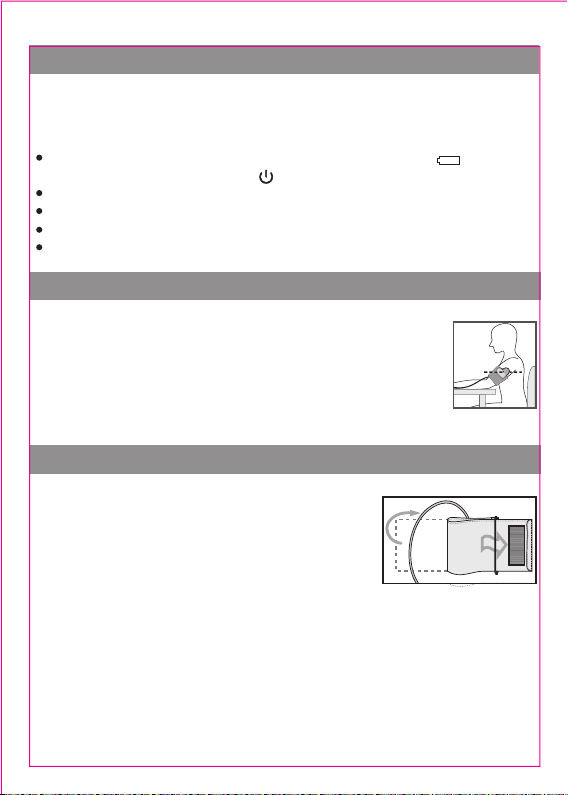
2. Take the measurement at room temperature in a quiet and stress-free
environment.
3. Do not move yourself or the unit during the measurement. Do not speak
during the measurement.
4. Blood pressure varies naturally depending on the time of day and is
affected by many factors. Blood pressure is usually highest at work and
reaches its lowest level during sleep.
5. Blood pressure measurements should be assessed by a physician or
trained healthcare professional who is familiar with your medical history. If
you use the unit and regularly record the results, keep your physician
informed of any changes in your blood pressure.
6. The performance of this device can be affected as severe arrhythmias
such as atrial or ventricular premature beats or atrial fibrillation are
presented during measurement.
7. The blood pressure measurements conducted with this unit are equivalent
to measurements obtained by a trained observer in accordance with the
values achieved using the cuff/stethoscope auscultation method and are
within the specified EN 1060-4 standard limits.
8. Patients with cardiovascular disease: take measurement under physician’s
instruction. Under no circumstances should you alter the dosage of any
drug prescribed by your physician!
9. Accurate measurement of blood pressure may be difficult in case of
arrhythmia, premature beat, atrial fibrillation, atreriosclerosis,
hypoperfusion, diabetes, pregnancy, nephropathy, weak pulse, or in
patients with obvious fluctuation of heart contraction rhythm. Consult a
physician to interpret your blood pressure readings.
6