10
CONNECTING THE DEVICE
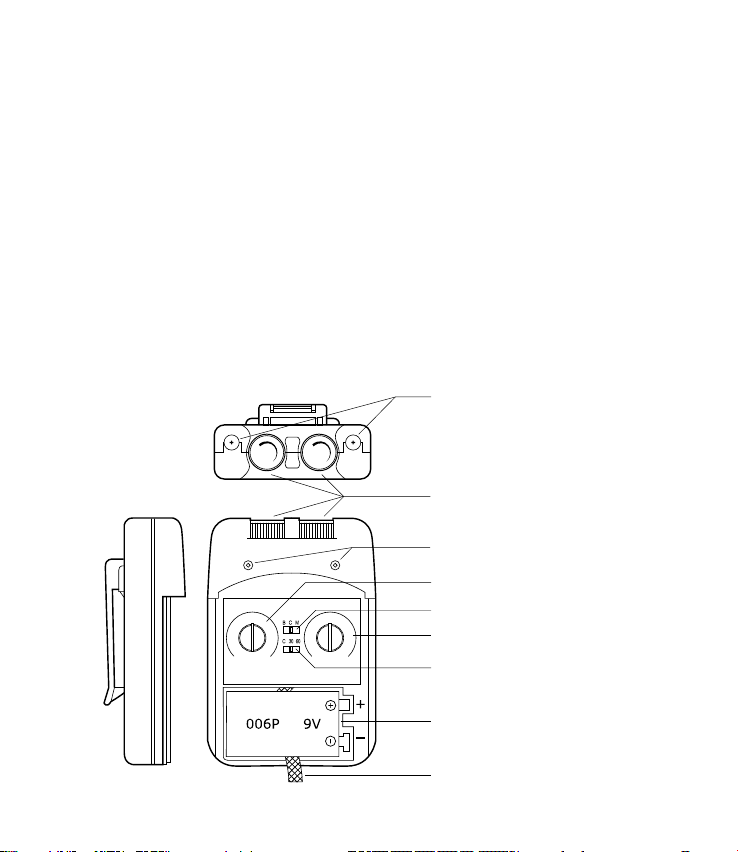
Insert battery
Turn the device to the OFF position before inserting or removing the battery.
When inserting the battery, ensure the battery polarity (+ and –) markings
match the markings on the device.
Prepare the Skin
Prepare the skin as previously described and according to the instructions
provided with your electrodes. Before attaching the electrodes, identify
the area that your physician / clinician has recommended for electrode
placement.
1. Connect the lead wires to the electrodes: connect the lead wires to the
electrodes before applying the electrodes to the skin.
WARNING: Ensure both Intensity Control Knobs for Channel 1 and 2 are
turned to the OFF Position (counterclockwise) before applying the electrodes.
2. Place electrodes on the skin: place the electrodes on the skin as
recommended by your physician / clinician.
3. Insert lead wire connector into the device: plug end of lead wire into the
channel output port (jack) to be used; push the plug in as far as it will go.
4. Select treatment settings: ensure your unit is still set to the proper
settings recommended by your physician / clinician.
5. Adjusting Channel Intensity Control: locate the Intensity Control Knob
(Channel 1 or 2) at the top of the unit. Slowly turn the Intensity Control
Knob clockwise until the stimulation is at the level recommended by your
physician / clinician (if you don’t feel anything, turn the Knob OFF then
ON again and carefully turn the Control Knob until you feel a tingling
or slight twitch under or around the electrodes). Always start with the
lowest setting and increase the intensity slowly.
If the stimulation levels are uncomfortable or become uncomfortable,
reduce the stimulation intensity to a comfortable level; or cease
stimulation and contact your physician.