Hanish water WaterCrest User manual

Owner’s Manual
2015

Thank you for your interest in Hanish Water. Whether you are just moving
into your first home, or you are looking to replace your existing water treatment
system with something different, we welcome you with open arms.
The modern water treatment industry has been in existence for almost 100
years now, and at no time in it’s history has it ever been so exciting. But yet it is
still in it’s infancy. The industry is also in the midst of it’s first real evolutionary
period. Why? Because every company in the industry is trying their hardest to go
GREEN with old fashioned technology. Don’t believe a word of it! There’s only
one truly green system on the market today,,,,,, the
WaterCrest
by Hanish
Water. Like you, we’re tired of hearing about this green product, and that green
product, because in reality, they’re still the same old, same old, except someone
put a green leaf sticker somewhere on the product, or packaging and then
pronounced it “green”.
At any rate, for whatever reason, you have decided to dig a little deeper
in an effort to find something different. Well, you’ve found it! Hanish Water exists
to give you the consumer, simply the finest, most efficient water processing
systems in the world. Our products are unlike any in the global water treatment
industry today, of which we have spent a great deal of time and energy
developing and refining. We like to describe them as “Industry Disruptive
Technologies” because they are simply so over the top in terms of function,
aesthetics, engineering, and simplicity that they can’t be matched on any level.
The primary objective of Hanish Water is to design the finest line of water
processing systems available on the planet today, and tomorrow. This company
was built for you by people who have spent their lives in the water treatment
industry.
Welcome to the best that the water treatment industry has to offer. Welcome
to Hanish Water.
Sincerely,
Chris J. Hanish
CEO & President

Introduction And Basic Water Chemistry
Water (H2O, HOH) is the most abundant molecule on Earth's surface,
composing of about 70% of the Earth's surface as liquid and solid state in
addition to being found in the atmosphere as a vapor. It is in dynamic equilibrium
between the liquid and vapor states at standard temperature and pressure. At
room temperature, it is nearly colorless with a hint of blue, tasteless, and
odorless liquid. Many substances dissolve in water and it is commonly referred to
as the universal solvent. Because of this, water in nature and in use is rarely
pure, and may have some properties different from those in the laboratory.
However, there are many compounds that are essentially, if not completely,
insoluble in water. Water is the only common substance found naturally in all
three common states of matter—for other substances, see Chemical properties.
Water also makes up 75 % of the human body.
Water is the chemical substance with chemical formula H2O: one molecule
of water has two hydrogen atoms covalently bonded to a single oxygen atom.
Water is a tasteless, odorless liquid at ambient temperature and pressure, and
appears colorless in small quantities, although it has its own intrinsic very light
blue hue. Ice also appears colorless, and water vapor is essentially invisible as a
gas.[3] Water is primarily a liquid under standard conditions, which is not
predicted from its relationship to other analogous hydrides of the oxygen family in
the periodic table, which are gases such as hydrogen sulfide. Also the elements
surrounding oxygen in the periodic table, nitrogen, fluorine, phosphorus, sulfur
and chlorine, all combine with hydrogen to produce gases under standard
conditions. The reason that oxygen dihydride (water) forms a liquid is that it is
more electronegative than all of these elements (other than fluorine). Oxygen
attracts electrons much more strongly than hydrogen, resulting in a net positive
charge on the hydrogen atoms, and a net negative charge on the oxygen atom.
The presence of a charge on each of these atoms gives each water molecule a
net dipole moment. Electrical attraction between water molecules due to this
dipole pulls individual molecules closer together, making it more difficult to
separate the molecules and therefore raising the boiling point. This attraction is
known as hydrogen bonding. Water can be described as a polar liquid that
dissociates disproportionately into the hydronium ion (H3O+(aq)) and an
associated hydroxide ion (OH−(aq)). Water is in dynamic equilibrium between the
liquid, gas and solid states at standard temperature and pressure (0°C, 100.000
kPa) , and is the only pure substance found naturally on Earth to be so.
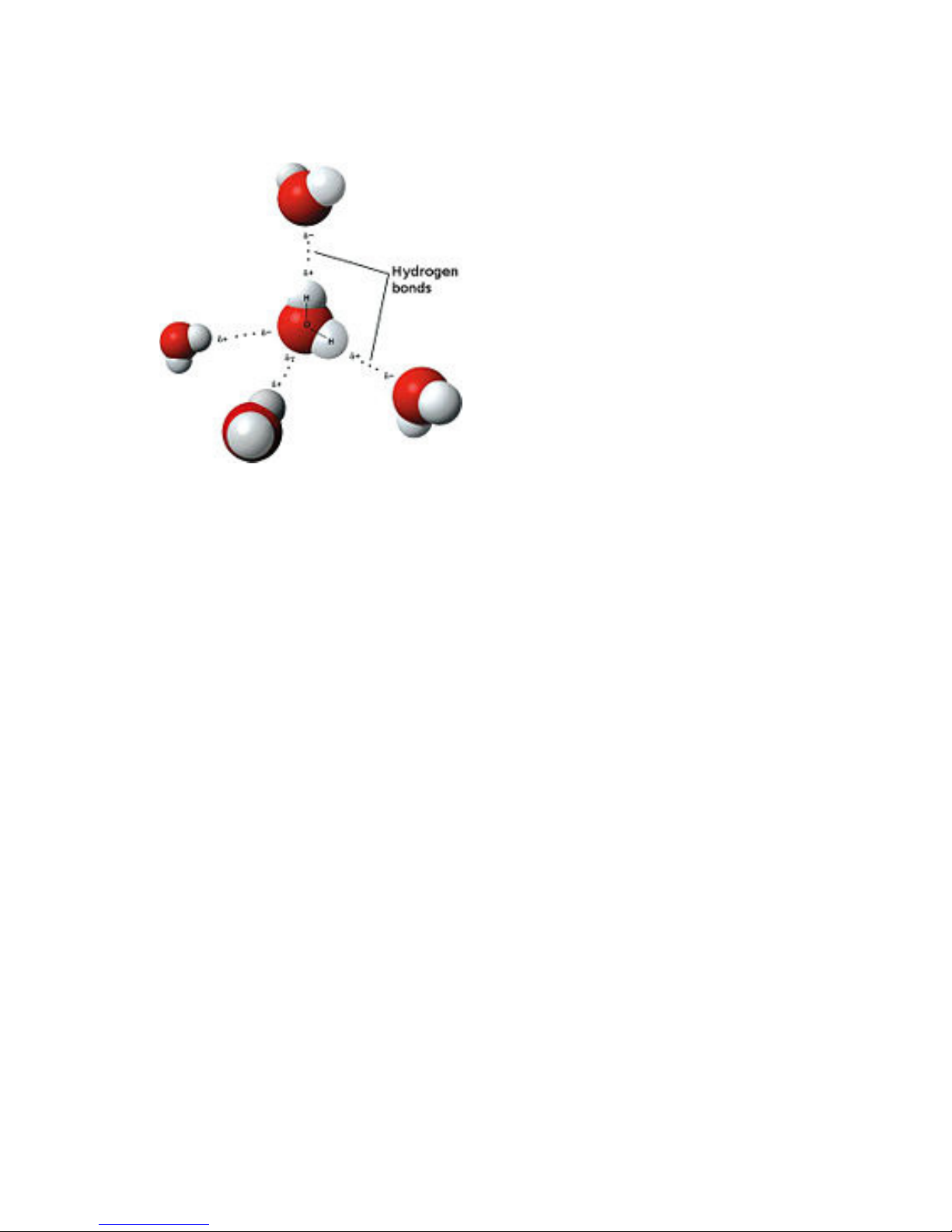
Dipolar nature of water
Model of hydrogen bonds between molecules of water
An important feature of water is
its polar nature. The water molecule
forms an angle, with hydrogen atoms at
the tips and oxygen at the vertex. Since
oxygen has a higher electro-negativity
than hydrogen, the side of the molecule
with the oxygen atom has a partial
negative charge. A molecule with such a
charge difference is called a dipole. The
charge differences cause water
molecules to be attracted to each other
(the relatively positive areas being
attracted to the relatively negative
areas) and to other polar molecules.
This attraction is known as hydrogen
bonding, and explains many of the properties of water. Certain molecules, such
as carbon dioxide, also have a difference in electro-negativity between the atoms
but the difference is that the shape of carbon dioxide is symmetrically aligned
and so the opposing charges cancel one another out. This phenomenon of water
can be seen if you hold an electrical source near a thin stream of water falling
vertically, causing the stream to bend towards the electrical source.
Although hydrogen bonding is a relatively weak attraction compared to the
covalent bonds within the water molecule itself, it is responsible for a number of
water's physical properties. One such property is its relatively high melting and
boiling point temperatures; more heat energy is required to break the hydrogen
bonds between molecules. The similar compound hydrogen sulfide (H2S), which
has much weaker hydrogen bonding, is a gas at room temperature even though
it has twice the molecular mass of water. The extra bonding between water
molecules also gives liquid water a large specific heat capacity. This high heat
capacity makes water a good heat storage medium.
Hydrogen bonding also gives water its unusual behavior when freezing.
When cooled to near freezing point, the presence of hydrogen bonds means that
the molecules, as they rearrange to minimize their energy, form the hexagonal
crystal structure of ice that is actually of lower density: hence the solid form, ice,
will float in water. In other words, water expands as it freezes, whereas almost all
other materials shrink on solidification.
An interesting consequence of the solid having a lower density than the
liquid is that ice will melt if sufficient pressure is applied. With increasing pressure
the melting point temperature drops and when the melting point temperature is
lower than the ambient temperature the ice begins to melt. A significant increase
of pressure is required to lower the melting point temperature —the pressure
exerted by an ice skater on the ice would only reduce the melting point by
approximately 0.09 °C (0.16 °F).

Water, The Perfect Solvent
Water is the perfect solvent due to its
polarity. Substances that will mix well and
dissolve in water (e.g. salts) are known as
"hydrophilic" (water-loving) substances, while
those that do not mix well with water (e.g. fats
and oils), are known as "hydrophobic" (water-
fearing) substances. The ability of a substance
to dissolve in water is determined by whether
or not the substance can match or better the
strong attractive forces that water molecules
generate between other water molecules. If a
substance has properties that do not allow it to
overcome these strong intermolecular forces,
the molecules are "pushed out" from the water,
and do not dissolve. Contrary to the common
misconception, water and hydrophobic
substances does not "repel", and the hydration
of a hydrophobic surface is energetically, but
not entropically, favorable.
When an ionic or polar compound enters water, it is surrounded by water
molecules (Hydration). The relatively small size of water molecules typically
allows many water molecules to surround one molecule of solute. The partially
negative dipole ends of the water are attracted to positively charged components
of the solute, and vice versa for the positive dipole ends.
In general, ionic and polar substances such as acids, alcohols, and salts
are relatively soluble in water, and non-polar substances such as fats and oils
are not. Non-polar molecules stay together in water because it is energetically
more favorable for the water molecules to hydrogen bond to each other than to
engage in van der Waals interactions with non-polar molecules.
An example of an ionic solute is table salt; the sodium chloride, NaCl,
separates into Na+cations and Cl-anions, each being surrounded by water
molecules. The ions are then easily transported away from their crystalline lattice
into solution. An example of a nonionic solute is table sugar. The water dipoles
make hydrogen bonds with the polar regions of the sugar molecule (OH groups)
and allow it to be carried away into solution.
This manual suits for next models
1
Table of contents
Popular Water Filtration System manuals by other brands

Atlantic Ultraviolet
Atlantic Ultraviolet Mighty Pure MP16A owner's manual

SunSun
SunSun CBG-500 Operation manual

Hayward
Hayward XStream Filtration Series owner's manual

Contech
Contech DownSpout StormFilter Operation and maintenance

Teka
Teka Airfilter MINI operating instructions

Wisy
Wisy LineAir 100 Installation and operating instructions

Schaffner
Schaffner Ecosine FN3446 Series User and installation manual

Pentair
Pentair FLECK 4600 SXT Installer manual

H2O International
H2O International H20-500 product manual

Renkforce
Renkforce 2306241 operating instructions

Neo-Pure
Neo-Pure TL3-A502 manual

STA-RITE
STA-RITE VERTICAL GRID DE FILTERS S7D75 owner's manual





