Hikoneb 908 DC User manual

EN
DE

1. Symbol Definitions 4
2. Important Safeguards 5-6
3. How the device works 7-8
4. Device Set-Up 8-10
5. Device Image and Components 10-11
6. Operating the device 12
7. Device Settings 13-16
8. Troubleshooting 16-17
9. Maintenance, Repair and Disposal 17-18
10.Cleaning, Disinfection, Sterilization 18-19
11. Accessories 20-21
12.Technical Specifications 21-22
13.Electromagnetic Conformity 22-24
Table of contents

1. Symboldefinition 26
2. Wichtige Sicherheitshinweise 27-30
3. Das Gerät im Überblick 30-31
4. Bedienung des Hikoneb 908 DC 32-33
5. Abbildung Hikoneb 908 DC und Komponenten 34-35
6. Inbetriebnahme des Hikoneb 908 DC 35-36
7. Geräteeinstellungen 37-40
8. Störungen/Fehlerbeseitigung 40-41
9. Reinigung, Desinfektion und Sterilisation 42-43
10.Wartung, Reparatur und Entsorgung 43-44
11. Zubehör 45
12.Technische Daten Gerät 46
13.Elektromagnetische Verträglichkeit 47-49
Inhaltsverzeichnis
Hikoneb | 908 DC
4
User Manual
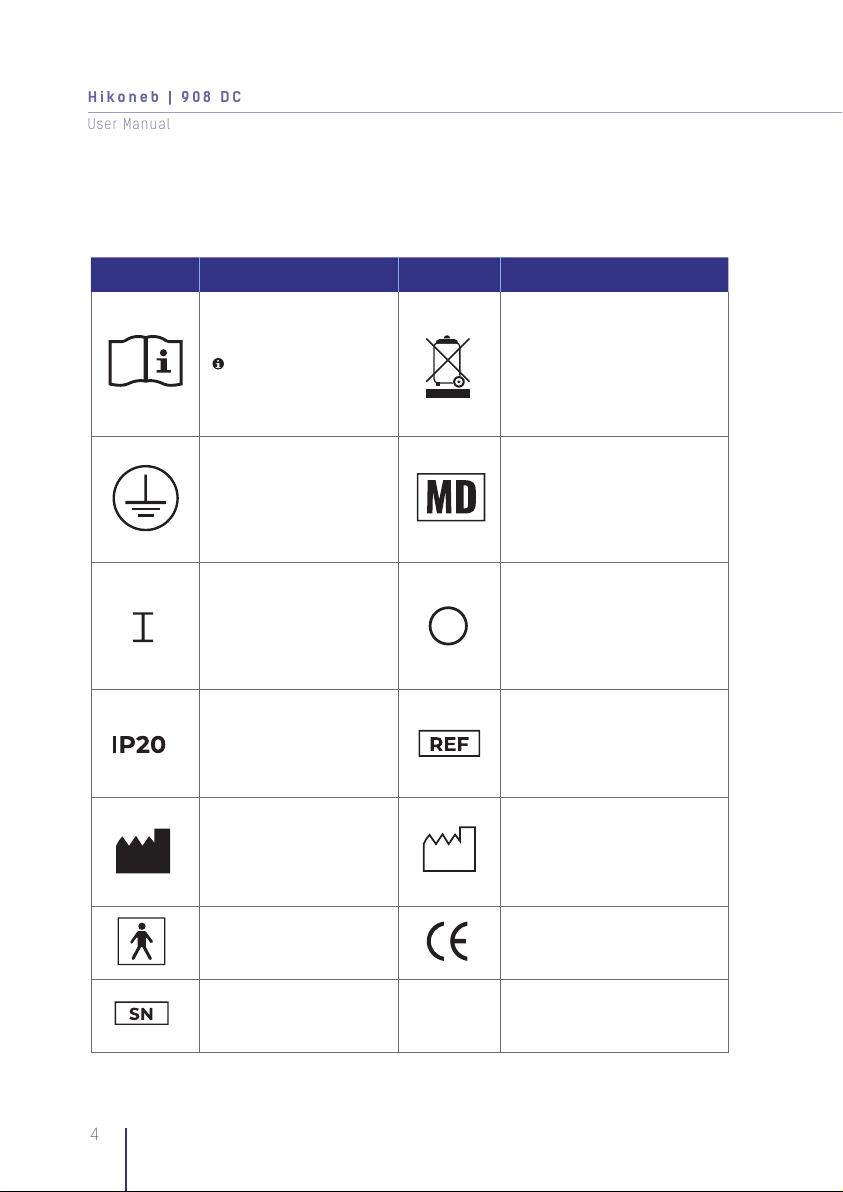
1. Symbol Definitions
It is mandatory to read and
understand the operating
instructions prior to use.
This symbol has a blue
background on the product
label
This device contains electronical
and/or electrical equipment
that must be recycled per EU
Directive 2012/19 EU-Waste
Electrical and Electronic
Equipment (WEEE)
Type of Protection. Ground
Conductor as per Class I Medical device
Indicates that this
control function places
the equipment into a fully
powered state
Indicates that this
control function places the
equipment into a fully
powered state
The device is protected
against solid objects of
12.5mm and more Item code
Manufacturer Date of production
Type B applied part Notified body
Serial Number

5
HN 908 DC_EN Rev. A
Hikoneb | 908 DC
User Manual
2. Important Safeguards
When using electrical products, especially when children are present, basic safety
precautions should always be followed.
Read all instructions before using. Important information is
highlighted by these terms:
DANGER – Urgent safety information for hazards that will cause serious injury or death.
WARNING – Important safety information for hazards that might cause serious injury.
CAUTION – Information for preventing damage to the product.
NOTE – Information to which you should pay special attention.
DANGER
To reduce the risk of electrocution:
• Always unplug this product immediately after using.
• Do not use while bathing.
• Do not place or store product where it can fall or be pulled into a bath tub or sink.
• Do not place in or drop into water or other liquid.
To reduce the risk of burns, electrocution, fire, or injury to persons:
•The device should never be left unattended when plugged in. Close supervision is
necessary when this product is used for or on near children or physically incapacitated
individuals.
•Use this product only for its intended use as described in this guide. Only use
attachments/spare parts recommended by the manufacturer.
•Do not nebulize home-therapy or aroma-therapy oils with this device.
•Do not use on ventilated patients.
Caution

Hikoneb | 908 DC
6
User Manual
•Do not use this product with a DC to AC converter, or with any AC voltage and/or
frequency other than specified.
•The Hikoneb 908 complies with EMC requirements conforming to IEC:60601-1-2.
Nevertheless, location should be considered with caution, when this product is used in
the vicinity of other electronic equipment where electromagnetic interference may be
an issue.
•Make sure that all parts are connected correctly, and that the dust filter is clean. A filter
which has changed its colour or used a period of 3 months, should be replaced.
•Bacteria filter should be replaced every 48 hours, in between patients and when it gets
dirty.
NOTE
•Dispose of any remaining medicines. Do not use again.
•Medicine cups are for single use only.
•Use original parts and accessories only.
•The device has been designed for inhalation using a mouthpiece or a face mask.
When only using the aerosol tube (for humidification purposes), there should be at least
10cm distance between a patient and the tube.
•Room/ambient temperature should not exceed 30°C when used with the heater
system.
•Only medical clean/sterile water should be used for nebulization. Medicines can be
nebulized as aqueous solution only.
•The nebulizer chamber is equipped with a maximum and minimum water level mark.
Water should be filled into the chamber between these two marks. If a sterile water
bottle is used, the gasket should be placed completely on the bottle lid, to prevent
flowing water from the chamber. In addition, make sure that the lid is tightly secured on
the sterile water bottle. After each use, this system should be cleaned, disinfected and
sterilized.
•The device should be used with original manufacturers air filter.
•Caution should be taken when removing the chamber, tubing/tube heater after
therapy, as these items may be very warm.
•Only authorized service personnel are allowed to open the device to conduct
troubleshooting and failure analysis. Do not try to open or repair the device by yourself.

7
HN 908 DC_EN Rev. A
Hikoneb | 908 DC
User Manual
3. How the device works
The Hikoneb 908 uses an ultrasonic frequency to turn a liquid medication into an
aerosol.
The device can be started and controlled by using push buttons. The base of the
nebulizer chamber contains a transducer, which causes the liquid in the chamber
to nebulize through its high-frequency oscillations. A blower located in the housing
conveys the aerosol through the patient tube to the patient for inhalation. This
driving air is passed through a bacteria filter before nebulization.
The Hikoneb 908 is equipped with an alarm system. An audible alarm is activated
when water in the nebulization chamber is below the minimum water level and the
device will automatically switch off.
Service Life
The expected service life of the device is 10 years.
Intended Use / Indication
The Hikoneb 908 DC has been manufactured for Ultrasonic Nebulizer Aerosol
treatment. It breaks water or liquid medicine into particles and produced
respirable water or medicine steam It is used for Asthma, Bronchitis, Pharyngitis
and Allergies and other respiratory diseases.
The Hikoneb 908 DC is used in hospital environments, hospital employees make
usage and maintenance operation. It is not suitable to connect to anesthesia
respiration system or ventilator systems. A doctor decides medicine type and
usage, a user cannot practice this treatment directly by himself/herself. The
devices are not support, patient monitoring and sterilized devices. The device has
no measurement function.
Even though high-volume ultrasonic nebulizers are not intended for direct patient
application, mouthpieces and aerosol masks can be used when administering
medications and substances intended to be inhaled (antibiotics, broncho-dilators
and for sputum induction)

Hikoneb | 908 DC
8
User Manual
Not suitable to be used with respiration masks. This warning is to avoid using with
protective masks such as N95. If a mask is to be used, then use aerosol/nebulizer
masks only.
Device intended use is for humidification of patient’s respiratory air. Room
humidification for medical and non-medical applications use is prohibited.
Manufacturer is not liable for product failure, all kind of damages and accidents
caused by non-intended use.
Contraindications
-Coughing, bronchospasm or allergic reactions.
-Patients unable to spontaneously breathe.
-Inability of the patient to cooperate accordingly.
Target Group
Spontaneously breathing patients from the age of 16 years for aerosol therapy.
4. Device Set-up
Assembly Instructions
1. Assemble the 5 feet mobile stand by using a 6mm Allen key (included) to connect
the five wheels and five feet. (26 & 1) with the black middle base connector (3) and
6mm Allen bolts. Screw together the main stand rods (6) and connect to the base with
a 6mm bolt and Allen key.
2. Connect the main unit bracket clamp (5) with the assembled stand rod (6) by its
clamp and Allen key. Connect three knurled plastic locking nuts (4) below the device,
to the device´s (5). The distance between the device and the ground should not exceed
60cm; a higher position can affect stability of the assembly.

9
HN 908 DC_EN Rev. A
Hikoneb | 908 DC
User Manual
3. Clip and fix the bottle holder (8) to the stand (6) with its screw.
4. If entraining oxygen, connect the oxygen adapter to device outlet, then connect the
bacteria filter (23) to the oxygen adapter (21) and connect the incoming oxygen to O2
adapter. Oxygen adapter must always be connected before the bacterial filter (between
device outlet and bacterial filter). If connected between bacterial filter and ventilation
tube, oxygen will enter to the system without any bacterial filtration.
5. If heated nebulization is desired, then place the heater (19) on chamber between the
lid and the aerosol tubing (15) and connect its power plug to the socket on the back of
the device.
BACK SIDE OF THE DEVICE
Heater Socket 30
Main Fuse 32
Power Input 31
Air Filter 33

Hikoneb | 908 DC
10
User Manual
6. Attach one end of the ventilation tube (16) to the bacteria filter (23) and the other end
to the nebulization chamber lid.
7. Connect the adjustable arm set (14) to the main stand rod. Connect the aerosol tube
(15) to the arm set’s black plastic clips. The support arm should tighten and secured
ensuring that the position of the aerosol tube is pointing upwards and straight, allowing
any collected aerosol to flow back into the chamber.
8. To start the aerosol therapy, place the required dose of medication into the
disposable medication cup (38), and connect to the chamber (11) and its lid (36). Locate
and lock the nebulization chamber in its place to the unit (22) by turning to the right.
5. Device Image and Components
1. Wheel
3. Middle base connector
4. Locking nuts
5. Bracket clamp
6. Stand rod
8. Bottle holder
11. Nebuliser chamber
14. Adjustable arm set
15. Aerosol tube
16. Ventilation tube
19. Heater
21. Oxygen adapter
22. Holding device
23. Bacterial filter (round)
26. Five feet
36. Nebuliser chamber lid
38. Medical cup

11
HN 908 DC_EN Rev. A
Hikoneb | 908 DC
User Manual
14
15
19
36
38
11
22
8
16
23
21
6326
1
5
4

Hikoneb | 908 DC
12
User Manual
6. Operating the device
Nebulization directly from the nebuliser chamber
• After the device is assembled use a sterilized nebulizer system and ensure that both,
air filter (33) and bacteria filter (23), are properly installed.
• Medication in doses between 5ml and 10ml can be nebulized.
• Remove the lid from the nebulization chamber (11) by turning it slightly back and forth
and pulling at the same time.
• Plug the conical medication cup (38) on the nebulization chamber lid (36) make sure
the water is between the minimum and maximum levels.
• Apply desired medication (max 10ml into the disposable medication cup (38).
• Connect the aerosol tube (15) and ventilation tube (16) to the chamber lid.
• Turn on the device using the ON/OFF switch which is located on the left side of the
control panel. The nebulization process will start.
Note: The distance between the device and the ground should not exceed 60 cm.
16
36
38
39
11
11. Nebulizer Chamber
16. Ventilation tube
36. Chamber lid
38. Medical cup
39. Transducer
13
HN 908 DC_EN Rev. A
Hikoneb | 908 DC
User Manual
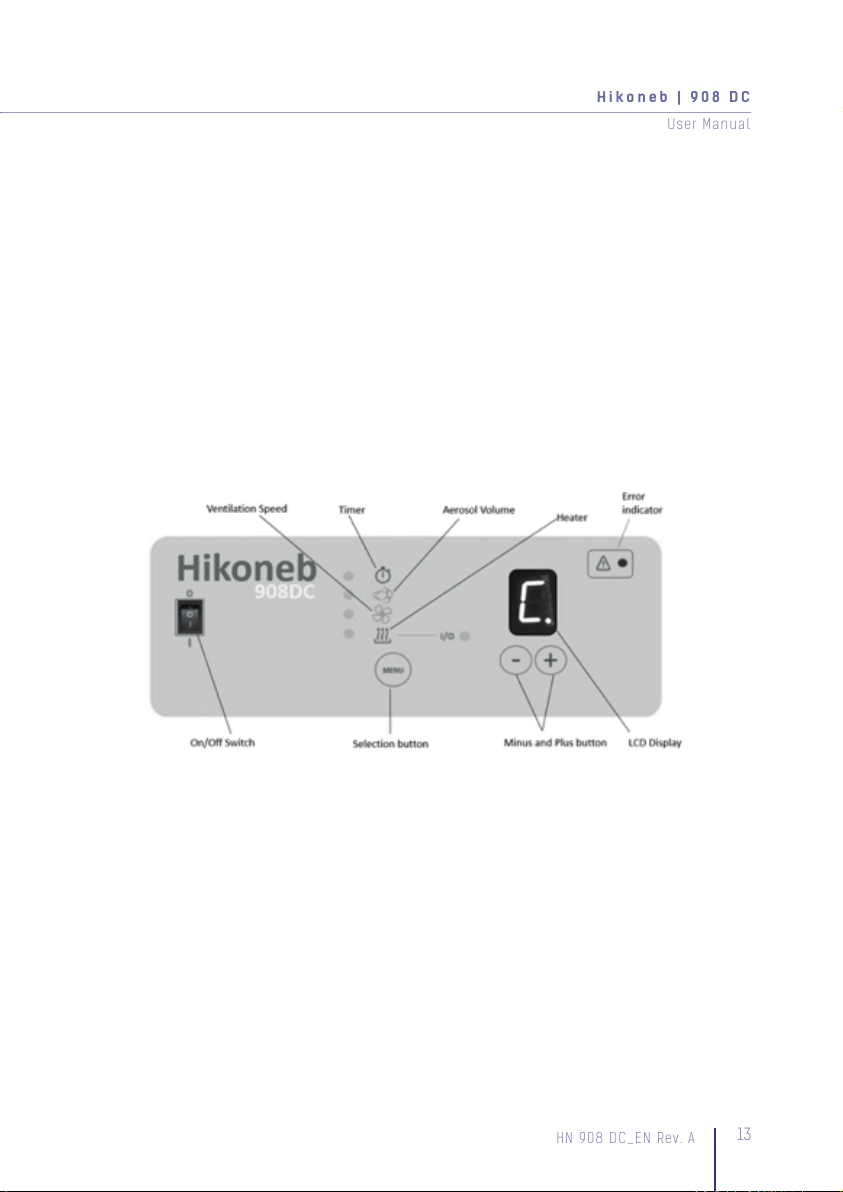
7. Device Settings
The Hikoneb 908 ultrasonic nebuliser has various menu settings in order to
accommodate individual patient requirements. These menu settings consist of a
Timer Setting, Aerosol Volume, Ventilation Speed and a Heater function. All settings can
be accessed via the “Menu”-button. A green LED indicates the position within the Menu.
Operation Panel
Timer Setting
By default, the Timer Setting is set to continuous operation C. By pushing the + button
the integrated timer can be set between 1 and 9, which is equal to 10 – 90 minutes. 0
stops the operation of the device, all other settings will remain. Once the Timer Setting
has been activated, the timer will count down (in steps of 10) to 0.

Hikoneb | 908 DC
14
User Manual
Aerosol Volume
By default, the Aerosol volume is set to 2. The Aerosol Volume density is adjustable in
steps between 1 and 4. By pushing the – or + button the Aerosol Volume produced, can
be increased or decreased.
Ventilation Speed
By default, the Ventilation Speed is set to 2. The Ventilation Speed is adjustable in steps
between 1 and 4. By pushing the – or + button the nebulization flow can be increased
or decreased.

15
HN 908 DC_EN Rev. A
Hikoneb | 908 DC
User Manual
Heater Function
By default, the Heater Function is set to 0. Pushing the + button will activate the heater.
An on/off LED (I/O) is illuminated when set, to let the user know that the Heater Function
has been activated.
Note
• Before practicing medicines, product information provided by the manufacturer
should be examined carefully. A qualified physician should administer/prescribe the
dosage of medicine to be used.
•Only liquid medicine/solutions shall be used.
•Maximum medication 10 ml.
• Therapy Process and respiration period should be determined by a qualified physician.
•Disposable conical medicine cup is for single therapy/patient only.

Hikoneb | 908 DC
16
User Manual
Note
• Due to its chemical characteristics, the nebulization chamber, medication cup
and aerosol & ventilation tubes are not suitable for ether type lubricants, ketones,
hydrocarbons and cyclohexanone type substances.
Warning
Low Level Water alarm: If the water in the Nebulization Chamber falls below the lower
limit, the warning led will illuminate and the unit will alarm. When water is added, both
warning LED and alarm will deactivate.
8. Troubleshooting
Error Reason Solution
The device is not
operational,
On-Off button light is
not on
No mains Check plug, IEC connections and
fuses
On-Off button is on
but there is no aerosol
production
1. Transducer
2. O/C fuse 1.Replace transducer
2. Replace fuses
Red error LED is on Low Water Level
Nebulizing chamber not locked
on the device correctly
1. Add more water
2. Re-fit chamber ensuring correct
Aerosol production is
visible, but not coming
out of tubing
Blockage of patient tube due to
wrong positioning
Occluded Filter
Flow menu disabled
Faulty fan
Change position of the aerosol tube
to point upwards and straight
Replace the filter
Activate Flow setting
Replace Fan

17
HN 908 DC_EN Rev. A
Hikoneb | 908 DC
User Manual
Electric shock! Before replacing the fuses, disconnect the device from the mains.
The main fuse (2A) is located inside the IEC socket on the back of the device. Remove
the power cord from the device. To remove fuse holder, use a small flat screwdriver
pulling the holder forward. Replace fuse with the same fuse rating and replace fuse
holder by pushing back into position.
8a. Replacement of the main fuse.
Caution
9. Maintenance, Repair and Disposal
Looking after your device and keeping it in good condition will ensure the device lasts
the expected service life. To keep the device in good working order, we advise to clean
the device after every use, and would suggest functional testing every six months as
per the instructions detailed in this user manual. Should the device ever become faulty,
technical support maybe required.
Bacteria filters can be disposed of with normal domestic waste. Nebulization chamber,
mobile trolley wheels, aerosol tubing which include PC, ABS and PVC can also be
considered as normal waste. The outer casing of the device itself is constructed from
aluminium and would need to be recycled or disposed of under WEEE regulations.
Devices returned to the manufacturer for disposal, will be processed under WEEE
Regulations.
Bacteria Filter Replacement
Using the approved bacteria filter (19) is mandatory to prevent contamination during
aerosol therapy. The filter should be replaced after 48 hours of continuous use, or
1 week for non-continuous use. Low aerosol output is also a good indication of a
contaminated/occluded filter.

Hikoneb | 908 DC
18
User Manual
Transducer Replacement
Turn off the device, remove the Nebulization chamber (9) and take off the lid of
nebulization chamber. Undo the chromium-plated nut underneath the nebulization
chamber and remove the complete transducer assembly (41). Replace the new
transducer so that the shiny side is facing towards the nebulization chamber. Reapply
and tighten the chromium nut.
Note
The device will not operate if the transducer is not installed correctly. Also, whenever the
transducer has been replaced and before the chamber is connected in place, fill the
chamber with water and check for possible leaks.
Chromium-plated
Nut, transducer
installed
Transducer
Chromium-plated
nut
10. Cleaning, Disinfection, Sterilization
Cleaning
The device and 5 feet mobile stand can be cleaned with a mild detergent and damp
cloth. For special cleaning/disinfecting please follow the manufacturers cleaning
instructions detailed in this user manual.
NO parts should be exposed to excessive pressure during the cleaning process.
Pay specific attention to the transducer surface ensuring it is clean. Clean lime scale
residue by wiping, not scrubbing. Abrasive scrubbing can damage the transducer.

19
HN 908 DC_EN Rev. A
Hikoneb | 908 DC
User Manual
Disinfection
When disinfecting the device surface, the device’s surface, make sure that the mains
power cable is removed. Ensure that no liquid enters inside the device when cleaning.
Operate the device only after the disinfection has completely dried and evaporated. Use
only disinfection products consisting of aldehyde, ammonia and alcohol components.
Never use phenol derivative disinfections, as this will compromise and reduce the
service life of plastic parts.
Examples of surface disinfectants:
- Incidin® Plus *
- Incidin® Perfect *
- Antiseptica Kombi surface disinfectant *
- Antifect® FF *
- B 10 *
Examples of surface disinfectants:
- Cidex® AF
- Gigasept® FF *
* Registered trademarks of the respective manufacturers
The lifespan of the nebulizer chamber with lid and the quartz module is limited to 30
autoclaving cycles. The example disinfectants are recommendations from the device
manufacturer. The recommendation of the mentioned active ingredient bases (pH 4.5-
8) applies. This does not release the user from the need to consult a hygiene specialist
in individual cases or to test the components to be disinfected for compatibility with the
active ingredients.

Hikoneb | 908 DC
20
User Manual
Sterilization
Certain parts of the device and nebulizer chamber can be steam sterilized.
Steam sterilization is allowed only for the following parts up to 131°C:
Nebulizer Chamber (11)
Nebulizer Chamber lid (36)
Aerosol Tube (15)
Ventilation Tube (16)
Before sterilizing, ensure that any cleaning or disinfection residue is completely
removed. Once sterilized, please examine all parts for possible damage. Any damaged
part should be replaced before use.
ATTENTION: Disposable Medication Cup (38) is for single use only.
11. Accessories
Item code Description Figure
X000658 Air filter (pack of 2) 33
M000042 Nebulizer Chamber, complete
(includes lid and transducer) 11, 36, 39
M000436 1,7Mhz transducer 39
X000661 Bacterial filter (round) 23
X000659 Ventilation tube 16
X000660 Aerosol tube 15
X000634 Oxygen adapter 21
M000046 Heater 19
G-252-001 Power plug (3 prongs), EU
Other manuals for 908 DC
1
Table of contents
Languages: