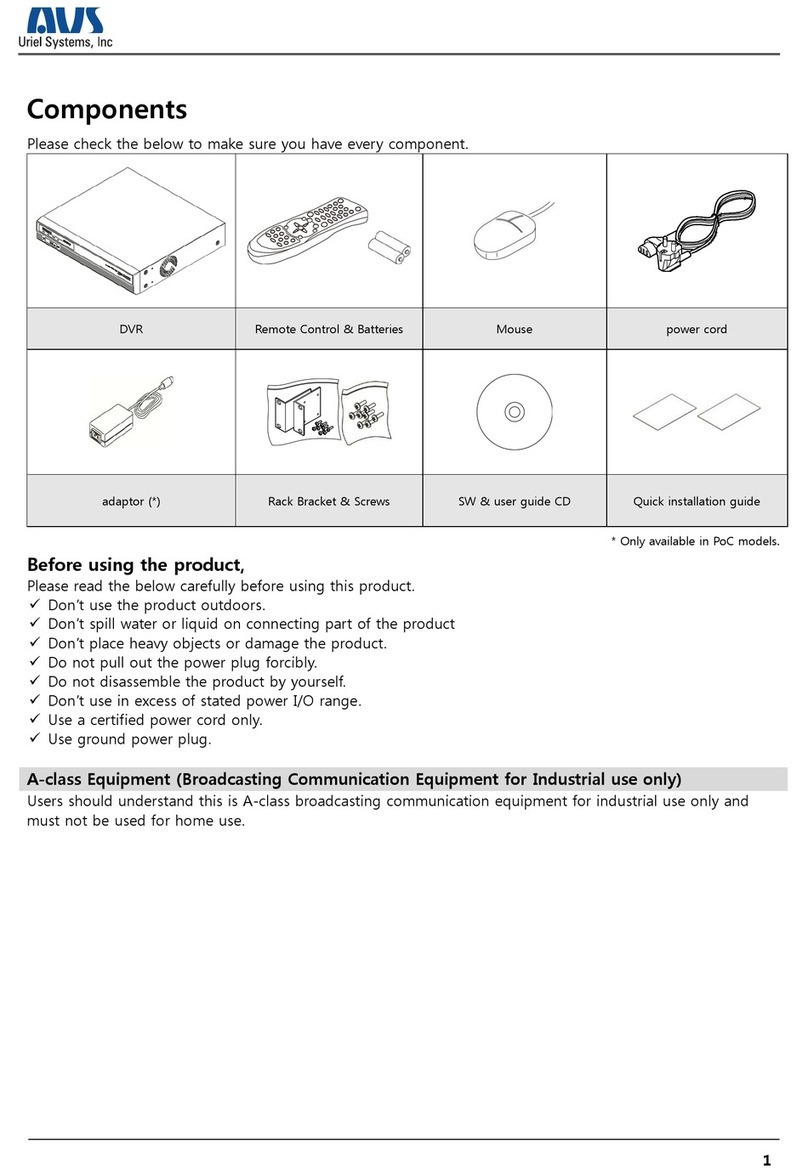
Hillrom Helion Main Unit R User manual

Instructions for use
Helion
Video Management System
ENGLISH
en-GB
Read the instructions for use carefully prior to using the
product and keep them safe for future reference.

This page is intentionally left blank.

Helion
80028681_002_C – 774259 – 2021-09-01 3
Manufacturer VIDEOMED S.r.l.
Via C. Battisti, 31/C
35010 Limena (Pd)
Italy
hillrom.com
VIDEOMED S.r.l. is a company of the Hill-Rom Holdings Group. The
manufacturer is referred to hereinafter as VIDEOMED.
Technical Customer Service The contact details for the current Technical Customer Service
hubs in the individual countries are listed on the Internet at
www.hillrom.com.
Information about the document Instructions for use
This document is identified by a code which indicates its version
and update status. The user is responsible for ensuring that the
most recent version is used.
This document applies to the following units sold:
The manual is provided by VIDEOMED S.r.l. in electronic PDF
format on digital media. A hard copy of the manual is available
upon request for qualified technical and medical personnel.
Telephone: +39 049 9819113
Fax: +39 0434 030689
Document number: 80028681
Language ID: 002
Version: C
Material number: 774259
Publication date: 2021-09-01
Product designation REF
Helion Main Unit R VR401111-1
Helion Main Unit VR401111-1ND
Helion Main Unit RD VR401111-1D
Helion Main Unit RSD VR401111-1DT
Helion Main Unit RS VR401111-1T
Helion Main Unit S VR401111-1TND
Helion Main Unit AR VR401111-2
Helion Main Unit ARD VR401111-2D
Helion Main Unit ARSD VR401111-2DT
Helion Main Unit ARS VR401111-2T
Helion Main Unit SSD R VR401111-3
Helion 4K VR401112
Helion 4K Plus VR401113
Helion Conference CM401326
Helion Rack (115V) AC500920K
Helion Rack (230V) AC500920K-2

Helion
80028681_002_C – 774259 – 2021-09-01 5
PREFACE
All rights reserved. No part of this publication may be copied, distributed, translated into other languages
or transmitted by any electronic or mechanical means, including photocopying, recording or any other
storage and retrieval system, for other purposes that are not exclusively the buyer’s personal use,
without the manufacturer’s express written permission.
The Manufacturer is in no way responsible for the consequences resulting from any incorrect operations
carried out by the user.
PUBLISHER'S NOTE
This documentation is specifically intended for clinically trained system users.
The Publisher is in no way responsible for the information and data contained in this manual: all
information contained herein has been provided, checked and approved by the Manufacturer for
verification.
The Publisher is in no way responsible for any consequences resulting from incorrect operations carried
out by the user.
GENERAL CONSIDERATIONS
All operating instructions and recommendations provided in this manual must be complied with.
Clinical personnel must be trained in all operating procedures and safety standards prior to using the
system.
SIGNAL WORDS
Residual dangers that may occur while using the product are identified in the document by the use of a
signal word. The required safety measures and potential consequences of failure to take these are listed.
A corresponding signal word provides an indication of the severity of the danger:
© 2021 VIDEOMED S.r.l.
Signal word Meaning
DANGER The signal word indicates a dangerous situation that will immediately lead to death
or serious injury if no precautionary measures are taken.
WARNING The signal word indicates a dangerous situation that may lead to death or serious
injury if no precautionary measures are taken.
CAUTION The signal word indicates a dangerous situation that may lead to moderate to slight
injury if no precautionary measures are taken.
NOTICE The signal word indicates a dangerous situation that may lead to material damage or
damage to the environment if no precautionary measures are taken.

Contents
80028681_002_C – 774259 – 2021-09-01 7
Contents
1 System identification. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
1.1 Identification plates. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
1.2 Reference standards. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
1.3 Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
2 General preliminary information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
2.1 Recipients of the instructions for use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
2.2 Updates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
2.3 Language. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
2.4 Personnel qualifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
2.5 Symbols used in the instructions for use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
3 Safety information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
3.1 General safety warnings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
3.2 Electromagnetic compatibility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
3.3 Useful life of the system . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
3.4 Cleaning. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
3.4.1 Preparing the system. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
3.4.2 Cleaning the system. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
4 System description. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .22
4.1 Intended use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
4.2 Reasonably foreseeable misuse . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
4.3 Use in combination with other medical devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
4.4 Obligations and prohibitions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
4.4.1 Personnel prohibitions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
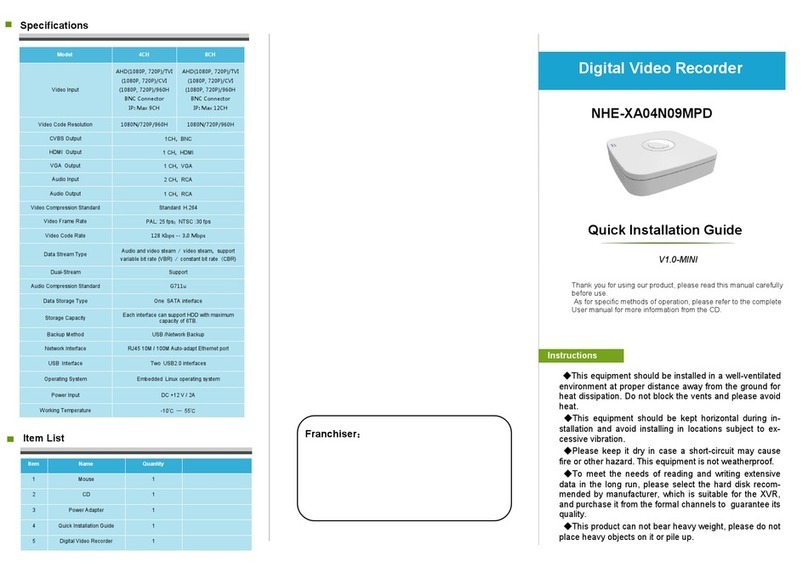
4.5 Technical data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
4.6 Measurement and weight layout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
4.7 System components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
4.7.1 Main unit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
4.7.2 Conference unit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
4.7.3 4K unit. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
4.7.4 4K Plus unit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
4.7.5 Control software. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
5 Operation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .38
5.1 First system start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
5.2 Preliminary checks. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
5.3 System start-up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
5.4 Connection to sources . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
5.5 System shutdown . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
5.6 System start-up/shutdown by remote button . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
6 User Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
6.1 General description of the user interface. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .41
6.2 Control touchscreen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
6.3 “Video Routing” function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
6.3.1 Live Preview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44
6.3.2 Quick Access - Recording. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46
6.3.3 Quick Access - Streaming. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 47
6.3.4 Ptz camera control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
6.3.4.1 Roomcam zoom adjustment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
6.3.4.2 Roomcam movement adjustment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
6.3.4.3 Save camera setting (Preset) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
6.3.4.4 Delete camera setting (Preset) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50

Contents
880028681_002_C – 774259 – 2021-09-01
6.3.4.5 Enable camera setting (Preset) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
6.4 “Recording” function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
6.4.1 Image data post-processing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53
6.4.2 Selecting the signals to be recorded. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53
6.4.3 Recording . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .54
6.4.4 Snapshot and video playback . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
6.4.5 Cutting video . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
6.4.6 Export images and videos . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 58
6.4.7 Delete images and videos . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .60
6.5 “Video Conference” function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
6.5.1 Selecting the signals to be sent by video conference . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 62
6.5.2 Removing the signals to be sent by video conference . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
6.5.3 Call recipient selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .64
6.5.4 Call start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
6.6 Additional functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
6.6.1 Patient data management . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .66
6.6.1.1 Selecting a patient in the list . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67
6.6.1.2 Entering a new patient . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68
6.6.1.3 Entering an emergency patient. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .69
6.6.1.4 Searching for a patient in a list . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70
6.6.1.5 Patient master data modification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70
6.6.1.6 Accessing the worklist . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 71
6.6.2 Check-List . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72
6.6.3 Preset. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73
6.6.3.1 Preset setting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 74
6.6.3.2 Enabling Preset. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 76
6.6.4 Multiview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
6.6.4.1 Multiview setting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 78
6.6.5 Audio control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 79
6.6.5.1 Volume adjustment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .80
6.6.5.2 Disabling microphones and audio. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 81
6.6.6 Surgical light management . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 82
6.6.7 Environmental control panel management . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 83
6.7 “Lock with PIN” function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .84
6.8 “Login” function. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 85
7 Disposal instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .86
8 Annex I - Getting started . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .87
8.1 Video Routing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
8.2 Multiview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
8.3 PTZ camera control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
8.4 Recording . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
8.5 Patient data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 88
8.6 Selecting the signals to be recorded . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 88

System identification
80028681_002_C – 774259 – 2021-09-01 9
1 System identification
1.1 Identification plates
Helion Video Management System units are fitted with identification plates. Each plate contains the
identification details of the unit which are to be quoted if required to VIDEOMED S.r.l.
The plates present are shown below:
Unit Image
Helion Main Unit R
Helion Main Unit
Helion Main Unit RD
Helion Main Unit RSD
Helion Main Unit RS
Helion Main Unit S
VR401111-1
Helion Main Unit R
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFNDJDK
AHFINKEOJEDJCJLKKAFK
ALCJNDFCLIKDLDDFJLNK
AIFAJJAIFOIGDGEBOAIK
ACOOAKMKECMCACMMGCEK
(01)00615521031572
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T3.15A
E464244
INPUT: 100-240V~1.1-2.0A 50/60 Hz
VR401111-1ND
Helion Main Unit
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFMDJDK
AHFINKEOJEDICMJINEEK
ALDINDFDLOIMAJECDDMK
AMFBJKCMLHAJLLCNBFJK
ACOKEKMOGKMOECEEAKEK
(01)00615521031671
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T3.15A
E464244
INPUT: 100-240V~1.1-2.0A 50/60 Hz
VR401111-1D
Helion Main Unit RD
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFNCJDK
AHFINKEOJEDJDJIEBEFK
AJDKNDFDKNLJIJJGLDMK
AMFBIMFBAFFHHMEDHDJK
ACOGAOKEKCMKIOMKICEK
(01)00615521031695
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T3.15A
E464244
INPUT: 100-240V~1.1-2.0A 50/60 Hz
VR401111-1DT
Helion Main Unit RSD
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFNCJDK
AHFINKEOJEDICNMOIHEK
ALAJNDFDKNNJBCEENDEK
AMFBIJAOKHBFAKJGIEIK
ACOKEOGEKKMCMCIEICMK
(01)00615521031688
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T3.15A
E464244
INPUT: 100-240V~1.1-2.0A 50/60 Hz
VR401111-1T
Helion Main Unit RS
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFNDJDK
AHFINKEOJEDIDJONEHEK
ALCLNDFDKKKNBKMGDDEK
AMFAJJGDKMKMPLPCAKIK
ACOOAOOMICMOIKKOMKMK
(01)00615521031664
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T3.15A
E464244
INPUT: 100-240V~1.1-2.0A 50/60 Hz
VR401111-1TND
Helion Main Unit S
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFNDJDK
AHFINKEOJEDIDMLMFNEK
AJEINDFCLKIDAIMPJLEK
AMFBIOEPICCKCCJBLLJK
ACOGEGKIACMGIOIIGKEK
(01)00615521031657
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T3.15A
E464244
INPUT: 100-240V~1.1-2.0A 50/60 Hz

System identification
10 80028681_002_C – 774259 – 2021-09-01
Helion Main Unit AR
Helion Main Unit ARD
Helion Main Unit ARSD
Helion Main Unit ARS
Helion Main Unit SSD R
Helion Conference
Helion 4K
Unit Image
VR401111-2
Helion Main Unit AR
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFMDJDK
AHFINKEOJEDJCJKGPEEK
AJGJNDFDLNMOGJOEMLMK
AMFAJOEEPDGHFFGMMHJK
ACOCACECKKEKAOMMKCEK
(01)00615521031640
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T3.15A
E464244
INPUT: 100-240V~1.1-2.0A 50/60 Hz
VR401111-2D
Helion Main Unit ARD
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFNCJDK
AHFINKEOJEDICIJPJNEK
AJGKNDFCLNPHAAENHLEK
AMFAJOCCIJJDNDPFDFJK
ACOCAGCACKMKMGKCCCEK
(01)00615521031633
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T3.15A
E464244
INPUT: 100-240V~1.1-2.0A 50/60 Hz
VR401111-2DT
Helion Main Unit ARSD
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFMDJDK
AHFINKEOJEDICLNKLEFK
AKFJNDFDKPJMCFFMNLEK
AMFAJJCHCGMNCCMDAHJK
ACOGECOOCKEOIGMIMKEK
(01)00615521031626
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T3.15A
E464244
INPUT: 100-240V~1.1-2.0A 50/60 Hz
VR401111-2T
Helion Main Unit ARS
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFNCJDK
AHFINKEOJEDJCKODNNFK
AKFKNDFCKPKFEMPFGLMK
AMFAJJEBFMDJKEFKPFJK
ACOGEGIMKKMOEOKGEKEK
(01)00615521031619
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T3.15A
E464244
INPUT: 100-240V~1.1-2.0A 50/60 Hz
VR401111-3
Helion Main Unit SSD R
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFMCJDK
AHFINKEOJEDIDPPJHEFK
AKHLNDFDKIOICNNODLEK
AMFBIJEKCNHENDKHIJJK
ACOCACGGACECMOOCICEK
(01)00615521031602
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T3.15A
E464244
INPUT: 100-240V~1.1-2.0A 50/60 Hz
CM401326
Helion Conference
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFMDJDK
AHFINKEOJEDJCLOIGCEK
ALBJNDFCLOKFBLONECFK
AMFBIJBGCOJJPIPEAGIK
ACOKEOCIKCMGMKEGAKMK
(01)00615521031886
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T1.6A
E464244
INPUT: 100-240V~1.1-2.0A 50/60 Hz
VR401112
Helion 4K
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFMCJDK
AHFINKEOJEDIDMKGGAEK
AJCLNDFCKLJGDDOBBLNK
AIFAIPHFOMNIPBCPIGIK
ACOCEOKAIKMGMOECOKEK
(01)00615521031596
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T1.6A
E464244
INPUT: 100-240V~0.2-0.48A 50/60 Hz

System identification
80028681_002_C – 774259 – 2021-09-01 11
CAUTION
It is strictly forbidden to remove the identification plates and/or
replace them with other plates. If the plates are damaged or
removed, the customer must notify the Manufacturer.
Helion 4K Plus
Helion Rack (230V)
(input voltage 220-240V)
Helion Rack (115V)
(input voltage 100-120V)
Unit Image
VR401113
Helion 4K Plus
116001
VIDEOMED MANAGEMENT SYSTEM
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHIALFMDJDK
AHFINKEOJEDIDMKAAJFK
ALAINDFDLPOONCBOMLFK
AIFBIJADCPMLEBLMJMIK
ACOKEOCAOKEOICIIKKEK
(01)00615521031589
(21)1234567890
(11)210811
7d
hillrom.co.uk
1234567890
2021-08-11
Made In Italy
FUSES RATING: 2X 250VAC - T1.6A
E464244
INPUT: 100-240V~0.2-0.48A 50/60 Hz
AC500920K-2
Helion Rack (230V)
800116
COMPONENT/ACCESSORY
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHKALFNCJDK
AHFINKEOJADJDJLDOOFK
AKAINDFCLNMIELOJICFK
AMFBJMBJFOHEHNJNGAJK
ACOKAKAOACMCAOEMEKEK
(01)00615521031893
(21)1234567890
(11)210903
7d
hillrom.co.uk
1234567890
2021-09-03
Made In Italy
FUSES RATING: 2X 250VAC - T6.3A
E464244
INPUT: 220-240V~ 2.6-2.9A 50/60 Hz
AC500920K
Helion Rack (115V)
800116
COMPONENT/ACCESSORY
Videomed srl
Via Cesare Battisti 31/C
35010 Limena, Italy
ALGOAOAIBKHKALFMCJDK
AHFINKEOJADIDNJBGGEK
ALGINDFDKJNOCIAJHKFK
AIFBJIGDGKJKLNDNFOIK
ACOGIOAMKCMKIKOKCKMK
(01)00615521031909
(21)1234567890
(11)210903
7d
hillrom.co.uk
1234567890
2021-09-03
Made In Italy
FUSES RATING: 2X 250VAC - T10A
E464244
INPUT: 100-120V~ 5.3-6.3A 50/60 Hz

System identification
12 80028681_002_C – 774259 – 2021-09-01
1.2 Reference standards
VIDEOMED S.r.l. declares that the Helion Video Management System complies with the specific
standards of the medical sector.
Legislation and rules applicable to the United States of America (USA):
Standard Description
21 CFR Part 820 Quality System Regulation
21 CFR Part 821 Medical Device Tracking Requirements
21 CFR Part 803, 806, 807 Medical Device Reporting,
Medical Device Reports of Corrections and Removals,
Establishment Registration and Device Listing for Manufacturers
and initial Importers of Devices
21 CFR Part 801 Labeling
19 CFR Part 134 Country of origin marking.
AAMI / ANSI / ISO 14971:
2007/(R)2010
Medical Devices - Application Of Risk Management To Medical
Devices
AAMI / ANSI / IEC 62304:2006 Medical Device Software - Software Life Cycle Processes
ANSI AAMI IEC 62366-1:2015 Medical devices - Part 1: Application of usability engineering to
medical devices
AAMI / ANSI HE75: 2009 Human Factors Engineering - Design Of Medical Devices
AAMI / ANSI ES60601-1:
2005/(R)2012 and A1:2012,
C1:2009/(R)2012
and A2:2010/(R)2012
Medical electrical equipment - part 1: general requirements for
basic safety and essential performance (IEC 60601-1:2005, Mod).
(General II (ES/EMC))
(U.S. Identical Adoption of Standard IEC 60601-1-2 Edition 4.0
2014-02
AAMI / ANSI / IEC 60601-1-2:2014 Medical electrical equipment -- part 1-2: general requirements for
basic safety and essential performance -- Collateral standard:
Electromagnetic disturbances -- Requirements and tests.
(General II (ES/EMC))
AAMI / ANSI / ISO 15223-1:2016 Medical Devices - Symbols To Be Used With Medical Device
Labels, Labelling And Information To Be Supplied - Part 1: General
Requirements
ISO 7010: Second edition 2011-06-01,
including amendment 1 (2012)
through amendment 7 (2016)
Safety Signs
California Proposition 65 Safe Drinking Water and Toxic Enforcement Act of 1986

System identification
80028681_002_C – 774259 – 2021-09-01 13
Legislation and rules applicable to European Union (EU) countries:
1.3 Warranty
The complete warranty clauses are provided in the sales contract.
VIDEOMED S.r.l. assures system safety and functional reliability
provided that:
– the system is used, managed and repaired solely as described
in these instructions for use;
– installation, modification and repair are carried out exclusively
by VIDEOMED S.r.l. assistance services
– only spare parts and accessories authorised by the
Manufacturer are used;
– no structural changes are made to the devices.
The system status following system testing must be recorded in an
installation protocol. Commissioning is used as proof of the start
of the warranty period.
Further details may be provided in the commercial contract.
The conditions required by the commercial contract (should they
differ) take priority over what is stated in this section.
Standard Description
Regulation (EU) 2017/745 The regulation on medical devices amending Directive 93/42/
EEC will enter into force on 26 May 2021
93/42/EEC Medical Devices Directive (MDD) and f.m. 2007/47/EC
EN 1041:2008 Information supplied by the manufacturer of medical devices
EN ISO 13485:2016 Medical devices — Quality management systems
EN ISO 14971:2012 Application of risk management to medical devices
EN ISO 15223-1:2016 Symbols to be used with medical device labels, labelling and
information to be supplied — Part 1: General requirements
EN 60601-1:2006/A1:2013 General requirements for basic safety and essential performance
EN 60601-1-2:2015 General requirements for basic safety and essential performance
- Collateral standard: Electromagnetic compatibility
EN 60601-1-6:2010 General safety standards — Collateral standard: Usability
EN 62304:2006 + A1:2015 Medical Device Software — Software Life-cycle Processes
EN 62366-1:2015 Application of usability engineering to medical devices
WEEE 2012/19/EU Waste electrical and electronic equipment
RoHS 2011/65/EU Restriction of the use of certain hazardous substances in
electrical and electronic equipment

General preliminary information
14 80028681_002_C – 774259 – 2021-09-01
2 General preliminary information
2.1 Recipients of the instructions for use
The instructions for use of the Helion Video Management System
are intended for operators trained and authorised to operate it.
Operator training must be documented.
The instructions for use contain topics referring to correct use of
the system, so as to keep its functional and qualitative
characteristics unchanged over time. All information and warnings
concerning correct, fully safe use are also provided.
The instructions for use, like the CE conformity certificate, are an
integral part of the system and must always accompany it in case
of any movement or resale. The user is responsible for keeping this
documentation intact, so that it may be consulted throughout the
entire lifespan of the system.
2.2 Updates
VIDEOMED S.r.l. reserves the right to update the instructions for
use at any time by means of modifications and/or translations
without prior notice.
Contact the VIDEOMED S.r.l. Customer Service office to be
updated on the latest version of the instructions.
2.3 Language
The original instructions for use have been drawn up in Italian.
Any translations into additional languages must be made on the
basis of the original instructions.
The Manufacturer is responsible for the information contained in
the original instructions; translations in other languages cannot be
fully verified, therefore, if an inconsistency is detected, it is
necessary to follow the text in the original language or to contact
the VIDEOMED S.r.l. Customer Service office.

General preliminary information
80028681_002_C – 774259 – 2021-09-01 15
2.4 Personnel qualifications
Consult the following table in order to establish personnel skills
and qualifications:
2.5 Symbols used in the instructions for use
Qualification Description
Operator Natural or legal person (for example, a doctor or a hospital) who
owns and uses the Helion Video Management System.
They must provide a safe system and adequately instruct the
user in the intended and permitted use of the system.
User A suitably trained person who, thanks to their professional
qualification, is authorised to operate and use the Helion Video
Management System for the required activities. They are
responsible for correct and safe operation of the system and for
ensuring that it is used solely for the intended purpose.
Qualified Personnel Authorised persons who are generally employees of the manager
or have acquired their skills through professional training in the
medical sector, are able to evaluate their work and recognise
potential risks based on their professional experience and
knowledge of safety regulations. Where required, qualified
personnel must certify their qualifications through a valid
document.
Symbol Description
Symbol used to indicate the need to consult the instructions for
use prior to using the equipment.
Symbol of compliance with Regulation (EU) 2017/745 on medical
devices.
Equipotential: symbol for “potential equalisation”.
Protective earth (ground)
Connection point for the neutral conductor on PERMANENTLY
INSTALLED equipment
Caution - Refer to the operating instructions.
Symbol used to indicate the date of manufacture.

General preliminary information
16 80028681_002_C – 774259 – 2021-09-01
Symbol used to identify the manufacturer's name.
Crossed-out bin: this product must not be disposed of as
communal mixed waste, collect separately.
Symbol used to indicate the VIDEOMED material number.
Symbol used to indicate the serial number.
Symbol used to indicate a medical device.
Indicates the Unique Device Identification (UDI) code, composed
of UDI-DI (01) and UDI-PI ((11) date of production (21) serial
number).
MEDICAL — GENERAL MEDICAL EQUIPMENT
FOR ELECTRICAL SHOCK, FIRE AND MECHANICAL HAZARDS,
ONLY IN ACCORDANCE WITH ANSI/AAMI ES60601-1 (2005) +
AMD 1 (2012), CAN/CSA-C22.2 No. 60601-1 (2014)
Consult instructions for use (IFU). A copy of the IFU is available on
this website. A printed copy of the IFU can be ordered from
Hillrom for delivery within 7 calendar days.
Symbol Description
hillrom.co.uk
7d

Safety information
80028681_002_C – 774259 – 2021-09-01 17
3 Safety information
3.1 General safety warnings
The Helion Video Management System must be used by suitably
trained personnel.
DANGER
ELECTRIC SHOCK FROM DAMAGED MAINS POWER CABLE!
Check the mains power cable before connecting it and do not
use it if it has been crushed or the insulation is damaged.
DANGER
ELECTRIC SHOCK FROM PRESENCE OF EXPOSED LIVE PARTS!
It is also recommended to periodically check the integrity of the
parts of the device, to check for the presence of exposed parts
following an impact or fall and to avoid using the device in case
of damage to the structure or to its components.
WARNING
This product can expose you to chemicals including lead and
Di(2-ethylhexyl) phthalate (DEHP), which are known by the State
of California to cause cancer, and lead and Di(2-ethylhexyl)
phthalate (DEHP), which are known by the State of California to
cause birth defects or other reproductive harm. For more
information, visit www.P65Warnings.ca.gov.
CAUTION
All safety information must be complied with to ensure safe use
of the Helion Video Management System.
CAUTION
To avoid complications due to electrostatic balancing charges
between parts of the device and the patient, the user must not
simultaneously touch the metal parts of the system and the
patient.
CAUTION
RISK OF CONTAMINATION AND INFECTION OF THE PATIENT!
Free particles hidden in worn parts may end up in open wounds.
A monitor with a damaged surface cannot be used in a medical
environment. If the control screen is mounted on a suspension
system, do not place the control screen over the sterile
operating area during medical use.

Safety information
18 80028681_002_C – 774259 – 2021-09-01
CAUTION
COMPULSORY MEASUREMENT OF DISPERSAL CURRENTS
It is necessary to measure leakage currents with circuits
downstream of the Helion system open. Otherwise, the leakage
currents of these circuits will be added to those of the Helion
system.
3.2 Electromagnetic compatibility
The Helion Video Management System supplied contains
electronic components subject to Electromagnetic Compatibility
regulations, which are affected by conducted and radiated
emissions.
The emission values comply with regulatory requirements thanks
to the use of components compliant with the Electromagnetic
Compatibility Directive, suitable connections and the installation
of filters where required.
The Helion Video Management System is thus compliant with the
Electromagnetic Compatibility (EMC) directive.
CAUTION
Any maintenance activities on the electrical equipment that are
carried out in a non-compliant manner or incorrect replacement
of components may compromise the efficiency of the solutions
adopted.
The Helion product is a Class A electromedical device according to
IEC 60601-1-2 (CISPR 11). It is suitable for use in a specific
electromagnetic environment. The customer and / or user of the
product must ensure that it is used in an electromagnetic
environment as described below.
Emission Test Compliance Electromagnetic Environment Guide
Radiated and conducted RF
emissions
CISPR 11
Group 1 The Helion system only uses RF (radio-
frequency) energy for its internal operation.
Therefore, the RF emissions are very low and
should not cause interference in adjacent
electronic devices.
Class A Helion is suitable for use in all buildings,
except for residential buildings and those
directly connected to the public low-voltage
power supply network that supplies buildings
used for residential purposes.
Harmonic emissions
IEC 61000-3-2
Not applicable
Voltage fluctuations / flicker
emissions IEC 61000-3-3
Not applicable

Safety information
80028681_002_C – 774259 – 2021-09-01 19
Guidance and manufacturer's declaration - Electromagnetic immunity
The product is suitable for use in a specific electromagnetic environment. The customer and / or user of
the product must ensure that it is used in an electromagnetic environment as described below:
Immunity test IEC test level Compliance
level
Electromagnetic environment – guidance
Electrostatic
discharge
(ESD)
IEC 61000-4-2
±8 kV in contact
±2, ±4, ±8, ±15 kV
in the air
IEC 60601-1-2
Test level
The floor must be finished in wood, concrete
or ceramic tiles. If the floors are covered with
synthetic material, relative humidity must be
at least 30%. Temporary signal loss may occur
(for a few seconds).
Radiated
electromagnetic
fields
IEC 61000-4-3
3 V/m
from 80 MHz to
2.7 GHz
IEC 60601-1-2
Test level
Portable and mobile RF communications
equipment should be used no closer to any
part of the EUT, including cables.
Minimum distance: 30 cm.
Fast electrical
transients (burst)
IEC 61000-4-4
± 2 kV for power
lines
± 1 kV for input /
output lines > 3 m
IEC 60601-1-2
Test level
The quality of the mains supply must be
typical for a commercial and / or hospital
environment.
Pulses
IEC 61000-4-5
±0.5, ±1 kV
differential mode
±0.5, ±1, ±2 kV in
common mode
IEC 60601-1-2
Test level
The quality of the mains power supply should
be typical for a commercial or hospital
environment.
Conducted
disorders, induced
by RF fields
IEC 61000-4-6
3 V
150 kHz to 80 MHz
6 V
ISM frequencies
IEC 60601-1-2
Test level
Portable and mobile RF communications
equipment should be used no closer to any
part of the EUT, including cables.
Minimum distance: 30 cm.
Networkfrequency
magnetic field (50/
60 Hz)
IEC 61000-4-8
30 A/m IEC 60601-1-2
Test level
Power frequency magnetic fields should have
the characteristic levels for a typical location
in a standard commercial or hospital
environment.
Voltage dips, brief
interruptions and
voltage variations
on power input
lines
IEC 61000-4-11
10 ms – 0% a 0°,
45°, 90°, 135°, 180°.
225°, 270°, 315°
20 ms – 0% a 0°
500 ms – 70% a 0°
5 s – 0%
IEC 60601-1-2
Test level
The quality of the mains voltage supply should
be typical for commercial or hospital
environments. If the user of the appliance
requires it to continue operating even when
the mains power supply is interrupted, it is
recommended to power the appliance with
an uninterruptible power supply (UPS) or
batteries.

Safety information
20 80028681_002_C – 774259 – 2021-09-01
Guidance and manufacturer's declaration - Range and frequency level: RF wireless communication
equipment
3.3 Useful life of the system
Provided that all applicable safety and maintenance regulations
are strictly observed, the video management system has been
designed to guarantee a useful life of 8 years.
The life cycle includes guaranteeing the functionality of the
product in accordance with the specific instructions for use,
supply of assistance service and the availability of spare parts;
VIDEOMED applies a certified quality management system in
accordance with EN ISO 13485 to all its business processes which
guarantees:
–highestquality;
– product and accessory reliability;
– ease of use;
– functional design;
– optimisation for the intended purpose.
Test frequency
(MHz)
Modulation Minimum immunity
level (V / m)
Applied immunity
level (V / m)
385 ** Pulse modulation: 18 Hz 27 27
450 * FM + 5 Hz deviation: 1 kHz sine
** Pulse modulation: 18 Hz
28 28
710
745
780
** Pulse modulation: 217 Hz 9 9
810
870
930
** Pulse modulation: 18 Hz 28 28
1720
1845
1970
** Pulse modulation: 217 Hz 28 28
2450 ** Pulse modulation: 217 Hz 28 28
5240
5500
5785
** Pulse modulation: 217 Hz 9 9
This manual suits for next models
30
Table of contents