Hitachi Aloka Medical UST-9121 User manual

i
MN1-1156 Rev.13MN1-1156 Rev.14
Electronic Convex Probe
UST-9121
Instruction Manual
MN1-1156 Rev.14

MN1-1156 Rev.13
ii

iii
MN1-1156 Rev.13
This is an instruction for model UST-9121, an ultrasound probe.
Read the manual carefully before using the instrument. Take special note of the items in section 1, "Safety
Precautions".
Keep this manual securely for future reference.
The CE mark on the probe indicates that this probe is valid when it is connected to equipment bearing the CE
mark that is specied as available in section 2 of this document. Therefore, if a probe bearing the CE mark is
connected to equipment that is specied as available but does not have a CE mark, part of this instruction
manual may not apply.
Symbols used in this document
The terms below are used in the safety information provided to prevent hazards and injuries to the operator
or patients. The severities of the hazard and injury that can occur when failing to observe the displayed
safety information are indicated in four levels: "Danger", "Warning", "Caution" and "Note".
Danger
Indicates an imminently hazardous situation which, if not avoided, will result in death or serious injury to
the operator or patient.
Warning
Indicates a potentially hazardous situation which, if not avoided, could result in death or serious injury to
the operator or patient.
Caution
Indicates a potentially hazardous situation which, if not avoided, may result in minor or moderate injury
to the operator or patient, or property damage only.
Note
Indicates a strong request concerning an item that must be observed in order to prevent damage or
deterioration of the equipment and also to ensure that it is used efciently.
The type of safety information is indicated by the symbols below.
This symbol means attention is required.
This symbol means that the described action is prohibited.
This symbol means the described action is mandatory.
Introduction

MN1-1156 Rev.13
iv
CONTENTS
1. Safety Precautions
1-1. Intended use......................................................................................................................1
1-2. Usage precautions.............................................................................................................1
1-2-1. Warnings and safety information ....................................................................................2
1-2-2. Washing, disinfection and sterilization precautions ............................................................3
1-2-3. Labels........................................................................................................................4
2. Specications and Parts name
2-1. Principles of operation....................................................................................................10
2-2. Specications..................................................................................................................10
2-3. Performance....................................................................................................................11
2-4. Names of each parts........................................................................................................11
2-5. Environmental conditions...............................................................................................12
2-5-1. Operating environmental conditions..............................................................................12
2-5-2. Storage environmental conditions.................................................................................12
2-6. Classication of ME equipment .....................................................................................12
3. Preparations for Use
3-1. Start up check .................................................................................................................13
3-1-1. Visual check .............................................................................................................13
3-1-2. Verication of washing, disinfection and sterilization.......................................................13
3-1-3. Verication of operation..............................................................................................13
4. Usage
4-1. Operation ........................................................................................................................15
4-2. Connecting to the ultrasound diagnostic instrument ......................................................16
4-3. Removing from the ultrasound diagnostic instrument ...................................................17
4-4. Precautions when performing puncture operations ........................................................18
4-5. Actions to be taken when an abnormal state is detected.................................................19
4-5-1. Ensuring safety of patients ..........................................................................................19
4-5-2. Handling the instrument..............................................................................................19

v
MN1-1156 Rev.13
5. Washing, Disinfection and Sterilization
5-1. Washing ..........................................................................................................................22
5-1-1. Probe tip ..................................................................................................................22
5-1-2. Cable and connector...................................................................................................22
5-2. Disinfection ....................................................................................................................23
5-2-1. Chemical disinfection.................................................................................................23
5-2-2. Gas disinfection ........................................................................................................24
5-3. Sterilization.....................................................................................................................25
5-3-1. Ethylene oxide gas (EOG) sterilization ..........................................................................25
5-3-2. STERRAD®sterilization.............................................................................................26
5-3-3. Liquid sterilization.....................................................................................................27
6. Storage
6-1. Actions before storing the probe.....................................................................................29
6-2. Environmental conditions for storage.............................................................................29
7. Moving and Transporting
7-1. Moving and transporting ................................................................................................31
7-2. Preparing the probe for moving......................................................................................31
7-3. Packing for transportation ..............................................................................................31
7-4. Environmental conditions during transportation ............................................................31
8. Periodic Inspection
8-1. Safety tests......................................................................................................................33
8-2. Testing of measurement tolerances.................................................................................34
8-2-1. Conducting tests ...........................................................................................................34
8-2-2. Result judgment............................................................................................................34
9. Conguration
9-1. Standard conguration....................................................................................................35
9-2. Options............................................................................................................................35
10. Disposal of the Device .......................................................................................................37
This Instruction Manual contains the main body of 38pages and 5pages until the CONTENTS.

MN1-1156 Rev.13
vi

-1-
MN1-1156 Rev. 13
1. Safety Precautions
1-1. Intended use
This probe is intended for use by a doctor or other qualied operator when placed to direct contact with the
skin making ultrasonic observations of surrounding organs.
Caution
Do not use this equipment for other than its intended purpose.
Use for other purposes can cause burns or other injuries to the patient or operator.
1-2. Usage precautions
The terms below are used in the safety information provided to prevent hazards and injuries to the operator
or patients. The severities of the hazard and injury that can occur when failing to observe the displayed
safety information are indicated in four levels: "Danger", "Warning", "Caution" and "Note".
Danger
Indicates an imminently hazardous situation which, if not avoided, will result in death or serious injury to
the operator or patient.
Warning
Indicates a potentially hazardous situation which, if not avoided, could result in death or serious injury to
the operator or patient.
Caution
Indicates a potentially hazardous situation which, if not avoided, may result in minor or moderate injury
to the operator or patient, or property damage only.
Note
Indicates a strong request concerning an item that must be observed in order to prevent damage or
deterioration of the equipment and also to ensure that it is used efciently.
The type of safety information is indicated by the symbols below.
This symbol means attention is required.
This symbol means that the described action is prohibited.
This symbol means the described action is mandatory.

-2-
MN1-1156 Rev. 13
1-2-1. Warnings and safety information
Warning
Follow the information in this manual and the documentation supplied with any equipment used
together with this probe.
Use that is not in accordance with the supplied documentation can result in a serious or moderate
injury, equipment breakdown, or physical damage that impairs operation.
Be sure to preparations for use.
Use the probe without noticing an abnormal condition can result in injury to the operator or patient.
If any abnormalities are noted on the probe in the start up check, immediately stop using it and
contact one of our ofces and/or distributor's ofces listed on the back cover.
See section 3-1 “Start up check”.
Do not use on the eyes.
This probe is not intended for use on the eyes. The acoustic output can have an adverse effect on
the eyes.
Do not attempt to disassemble, modify, or repair the probe.
Electric shock or other unforeseen accidents could result. Contact one of our ofces and/or
distributor's ofces listed on the back cover to request repair.
Clean, disinfect and sterilize before using the probe as necessary.
Perform properly wash, disinfect and sterilize after use.
Otherwise, there is a risk of infection. Note that the probe is not sterilized at the factory.
Before using the probe rst, be sure to wash, disinfect and sterilize it as required.
Wear medical gloves during examination.
Conducting examinations with the bare hands can expose the operator to a risk of infection.
Dispose of probes used for patients with Creutzfeldt-Jakob disease.
Otherwise, there is a risk of infection to the operator or patient. Currently, there are no methods
for washing, disinfecting and sterilizing probes which have been used on patients aficted by
Creutzfeldt-Jacob disease.
When using ultrasound contrast agent, follow the supplied documentation.
Unexpected accidents could result. Check the state of the patient and take appropriate precautions
to avoid side effects.
Do not use the probe fallen on to oor.
Otherwise, there is a risk of infection. Stop the operation and perform the procedure in section 8
"Periodic Inspection", section 5 "Washing, Disinfection and Sterilization" and section 3-1 "Start up
check".
Caution
Constantly check for anything abnormal about the patient’s condition and probe.
Continued use without noticing that an abnormal condition has occurred can result in an electric
shock and injury to the operator or patient. If an abnormal condition occurs, immediately move the
probe away from the patient and stop use of the probe.
The probe is vulnerable to damage by impact. Therefore, handle it with care.
There is a risk of damage to the probe when the probe is fallen or hit somewhere.
Do not use this probe with other equipment except for those specically approved in the manual.
Use with unapproved equipment can result in an electric shock, burn, or other injury to the patient
or operator and damage to the probe and the other equipment.

-3-
MN1-1156 Rev. 13
Scan for the minimum length of time necessary for the diagnosis and at the lowest suitable output.
Overuse can adversely affect the internal tissues of the patient.
For details about the acoustic output, please refer to the documentation supplied with the ultrasound
diagnostic instrument.
Regularly perform maintenance inspection and safety tests of the ultrasound diagnostic instrument
and probe.
If you use equipment for a long period of time, it can reduce the performance, or cause smoke or
re. If anything unusual occurs, immediately stop using it and contact one of our ofces and/or
distributor's ofces listed on the back cover.
Use, move and transport the probe under the environmental conditions specied in this manual.
Otherwise, it may be damaged.
See section 2-5 "Environmental conditions" and section 7-4 "Environmental conditions during
transportation".
1-2-2. Washing, disinfection and sterilization precautions
Warning
Wear protective gloves and other protective gear during washing, disinfection and sterilization.
Handling of the probe with your bare hands before disinfection or sterilization can result in an
infection.
After soaking in cleaning agents, thoroughly wash the probe with running water.
Residual chemicals can cause an adverse reaction on the bodies of the operator or patient.
After chemical disinfection and sterilization, thoroughly wash the probe with sterilized water.
Residual chemicals can cause an adverse reaction on the bodies of the operator or patient.
Perform aeration completely after gas disinfection and sterilization.
Residual gas can cause an adverse reaction on the bodies of the operator or patient.
Do not wash, disinfect or sterilize using procedures other than those specied in this manual.
Infection could result due to incomplete washing disinfection or sterilization. It can also result in
damage to the probe or reduced performance. The probe cannot withstand autoclave sterilization or
boiling and other types of sterilization at temperatures exceeding 60°C (140°F).
For details on the usage conditions of chemicals and sterilization procedures, refer to the
documentation supplied with the respective chemical or sterilization equipment.
Infection could result due to incomplete disinfection or sterilization.
This could also cause deterioration of the probe.
Caution
Do not place the probe tip in any liquids beyond the range
shown in the gure right.
The connector which liquid has intruded can cause the
malfunction of the probe and the ultrasound diagnostic
instrument. In this case immediately stop use and contact one
of our ofces and/or distributor's ofces listed on the back
cover. 21mm or less
Water or chemical solution

-4-
MN1-1156 Rev. 13
M
H
z
Label 3
Label 2
Label 1
Label 1
1-2-3. Labels
(1) Probe unit
Electronic convex probe mark
Frequency

-5-
MN1-1156 Rev. 13
Label 3
Label 2
STERRAD® sterilization compatibility mark
See section 5.
This equipment complies with Directive 93/42/EEC relating to
Medical Device.
IPX7 mark
See section 2-2, “Specications”.
Type BF applied part
Do not waste the instrument as general waste.
Comply with a local regulation.
See section 10.
Manufacturer
Model, Serial No.
Safety warning sign
Biohazard
See section 5.
Follow the instruction manual to operate this instrument.
If not avoided, may result in injury, property damage, or the
equipment trouble.
IPX7

-6-
MN1-1156 Rev. 13
Label A
(2) Storage case
Label B

-7-
MN1-1156 Rev. 13MN1-1156 Rev. 14
Label A
Model
Serial No.
Label B
2012
This equipment complies with Directive 93/42/EEC
relating to Medical Device.
DATE OF MANUFACTURE
(in case of 2012)
MANUFACTURER
2012

-8-
MN1-1156 Rev. 13

-9-
MN1-1156 Rev. 13
2. Specications and Parts name
2-1. Principles of operation
This probe and the ultrasound diagnostic instrument enable image diagnosis using ultrasonic waves. These
instruments operate under the principles described below.
(1) When an electric pulse signal is applied from the transmitter to the transducer of the probe, the
transducer operates by converting electrical vibrations to mechanical vibration energy for emitting
pulse-shaped ultrasonic waves into the body part contacting the transducer or into liquid or other
medium.
(2) The emitted ultrasonic waves are reected by boundaries with different acoustic characteristics (acoustic
impedance) within the body.
(3) The transducer is also used to receive reected ultrasonic waves. The transducer vibrates mechanically
due to the received ultrasonic vibrations and uses an electro-mechanical conversion operation to
convert the received mechanical vibrations to electric energy. The received echo is also converted to
electric signals and a brightness modulation operation is used to convert the electric pulses to shades of
brightness for forming an image.

-10 -
MN1-1156 Rev. 13
2-2. Specications
Application regions: Abdomen, general areas
Form of application to patient: Surface
Connectable instruments: SSD-1000, SSD-3500, SSD-4000, SSD-5500
Field of view: 120°
Frequency: 2.0 to 5.0 MHz
Cable length: 2.0 m
Weight: 930 g
Service life: Three years
Range of applied part: Ultrasonic irradiation area, see the section 2-4.
Parts treated as applied parts: Probe tip itself and 1 m of the cable near the probe tip.
IPX7 range: As shown in the gure below.
External dimensions: As shown in the gure below.
Unit: mm
Remarks
The dimensions and weight are within ±10% of the indicated values.
Cable length
IPX7 range
50
27.5 40.5
80.5

-11-
MN1-1156 Rev. 13
2-3. Performance
For measurement tolerances, operating tolerances and other data, refer to the instruction manual for the
ultrasound diagnostic instrument.
2-4. Names of each parts
Connector
This is the part that connects the ultrasound
diagnostic instrument and probe. Follow the
instructions in section 4-2.
Ultrasonic irradiation area
This incorporates an electronic convex
transducer.
Front mark
The round protrusion indicates the
direction of the front mark (direction
mark) on the image display.
Probe tip
This area is held during operation.
Cable
This cable propagates the ultrasonic signals
that are sent and received.
Caution
Do not pull, bend, twist, or apply excessive force to the cable.
The conductors may break and the cable may become unusable.
Do not subject the ultrasonic irradiation area to hard impact.
This could make the probe unusable.

-12-
MN1-1156 Rev. 13
2-5. Environmental conditions
Use and store the probe under the following conditions.
2-5-1. Operating environmental conditions
Ambient temperature: 10°C to 40°C
50°F to 104°F
Relative humidity: 30% to 75%
Atmospheric pressure: 700 hPa to 1060 hPa
Altitude: 3,000 m or less
2-5-2. Storage environmental conditions
Ambient temperature: –10°C to 50°C
14°F to 122°F
Relative humidity: 10% to 90%
Atmospheric pressure: 700 hPa to 1060 hPa
Caution
Avoid operating or storing the probe in the following locations.
• Locations exposed to water or other liquids
• Locations subject to adverse conditions such as air pressure, temperature, humidity, ventilation,
direct sunlight, dust, or air containing salt, sulfur, or other corrosive substances
• Locations where chemical substances are stored or where gases are generated
Storage in these locations can result in a breakdown or reduced performance.
Avoid rapid temperature change which may cause condensation. Avoid using in locations where
condensation or water droplets can form.
Condensation can occur when moving the probe from a cool location to a warm one. Use when
condensation has occurred can result in a breakdown or reduced performance.
2-6. Classication of ME equipment
• Classication based on degree of protection against electric shock . Type BF applied part
• Classication for protection against ingress of liquids ..................... IPX7 (Watertight equipment)
• Operation mode ................................................................................. Continuous operation
• Method of sterilization ...................................................................... See section 5 “Washing, Disinfection
and Sterilization”
For the range of applied parts, parts treated as applied parts and the range of IPX7, see section 2-2.

-13-
MN1-1156 Rev. 13
3. Preparations for Use
3-1. Start up check
3-1-1. Visual check
Visually check the probe tip, ultrasonic irradiation area, cable and connector.
If any holes, indentations, abrasion, cracks, deformation, looseness, discoloration, or other
abnormalities are found, do not use the equipment.
3-1-2. Verication of washing, disinfection and sterilization
Verify that washing, disinfection and sterilization are conducted according to the intended use.
3-1-3. Verication of operation
Connect to the ultrasound diagnostic instrument by following the instructions in section 4-2
“Connecting to the ultrasound diagnostic instrument” and check that the selected probe match the
convex display and the displayed frequency and check the image for errors.
Remarks
For details on the displayed screens, see the documentation supplied with the ultrasound diagnostic
instrument.
Warning
Be sure to preparations for use.
Using the probe without noticing an abnormal condition can result in injury to the operator or
patient. If an inspection nds an abnormal condition in the probe, immediately stop use and contact
one of our ofces and/or distributor's ofces listed on the back cover.
Caution
Do not use the probe if the selected probe and image do not match the frequency.
An incorrect acoustic output can result in burns or other injuries to the patient. Contact one of our
ofces and/or distributor's ofces listed on the back cover.

-14 -
MN1-1156 Rev. 13
Table of contents
Other Hitachi Aloka Medical Medical Equipment manuals
Popular Medical Equipment manuals by other brands
ARJO HUNTLEIGH
ARJO HUNTLEIGH citadel Instructions for use

Mind Media
Mind Media NeXus-10 MKII quick start
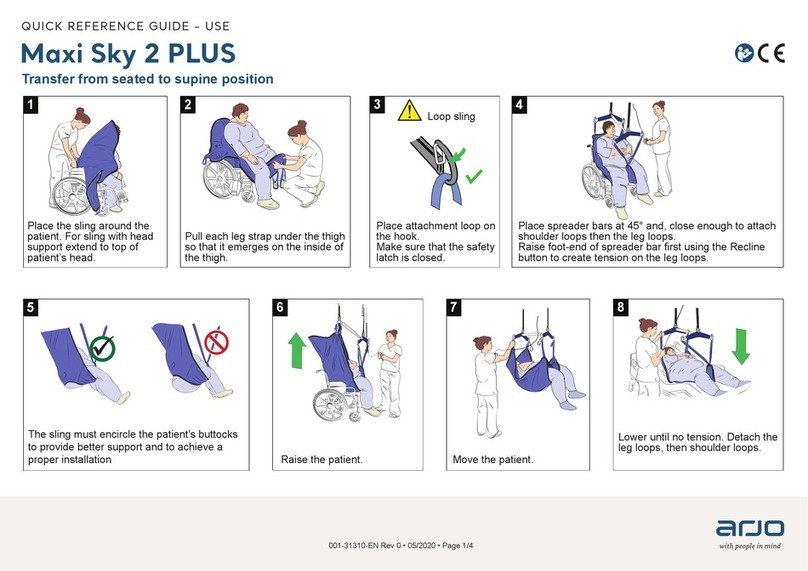
ARJO HUNTLEIGH
ARJO HUNTLEIGH Maxi Sky 2 Plus Quick reference guide

Etac
Etac Immedia GlideCushion Instructions for use

iSens
iSens i-Smart 300 VET Operator's manual

ARJO HUNTLEIGH
ARJO HUNTLEIGH FLOWTRON UNIVERSAL Instructions for use