Inovytec Ventway Sparrow User manual

VWSP-100 Civil Model
VWSP-900 Military Model
User Manual
Ventway Sparrow
Emergency and transport
ventilator
Document Number VWSP-5 - 3 October 2 18

Ventway Sparrow User Manual
Page 2 of 11
Important
This User Manual is subject to periodic review, update and revision.
Do not use a defective product. Do not repair this product or any of its parts.
If this device does not perform properly, contact an Inovytec representative.
The user of this product has sole responsibility for any malfunction that results
from improper use, faulty maintenance, improper repair, unauthorized
service, damage, or alteration by anyone other than Inovytec Medical
Solutions Ltd.
The safety, reliability, and performance of this device can be assured only
under the following conditions:
•The device has been used according to the accompanying operating
instructions.
•All fittings, extensions, readjustments, changes, or repairs have been
carried out by Inovytec Medical Solutions Ltd.'s authorized represen-
tatives.
No part of this publication may be reproduced, stored in a retrieval system or
transmitted in any form by any means, electronic, mechanical, photo
reproductive, recording or otherwise without the express prior written
permission of Inovytec Medical Solutions Ltd.
Inovytec Medical Solutions Ltd. reserves the right to change or improve its
products and accompanying technical literature without specific notice of
changes or improvements.
This product is protected by patents listed on the Inovytec website.

Ventway Sparrow User Manual
Page 3 of 11
Contact Information:
Inovytec Medical Solutions Ltd.
3 Hanagar St., POB 7282,
Hod-Hasharon 45 13 6, Israel
Tel: +972 9 779 41 35
Fax: +972 9 779 41 38
E-mail: [email protected]
Web Site: http://www.Inovytec.com
Disclaimer
Information provided by Inovytec Medical Solutions Ltd. is believed to be
accurate and reliable. However, Inovytec assumes no responsibility for the
use of such information, nor for any infringements of patents or other rights
of third parties, that may result from its use.
FDA Tracking equirements
U.S. Federal Law (21 CFR 821) requires the tracking of ventilators. Under this
law, owners of this ventilator must notify Inovytec Medical Solutions Ltd. if
this product is received; lost, stolen, or destroyed; donated or resold; or
otherwise distributed to a different organization. If any such event occurs,
contact Inovytec in writing with the following information:
Originator's organization – Company name, address, contact name, and
contact phone number
Model number, and serial number of the ventilator
Disposition of the ventilator (for example, received, lost, stolen,
destroyed, distributed to another organization), new location and/or
organization (if known and different from originator’s organization) –
company name, address, contact name, and contact phone number
Date when the change took effect
Please address this information to Inovytec Medical Solutions Ltd. at the
address given above.
PLEASE EAD THIS USE MANUAL BEFO E OPE ATING THE SYSTEM.

Ventway Sparrow User Manual
Page 4 of 11
Contents
1. About This User Manual .......................................................................................................... 9
1.1. TYPES OF WARNINGS, CAUTIONS AND NOTES ....................................................................... 9
1.2. GLOSSARY AND ABBREVIATIONS ................................................................................................... 1
2. Overview of System .............................................................................................................. 11
2.1. DESCRIPTION OF DEVICE ............................................................................................................. 11
3. Conditions for Use ................................................................................................................ 12
3.1. INTENDED USE ......................................................................................................................... 12
3.2. INDICATIONS FOR USE ................................................................................................................ 12
3.3. CONTRAINDICATIONS ................................................................................................................. 13
3.4. LIMITATIONS OF USE ................................................................................................................. 13
4. Safety ................................................................................................................................... 14
4.1. ELECTRICAL SAFETY ................................................................................................................... 14
4.2. EMC COMPLIANCE ................................................................................................................... 14
4.3. SAFETY INSTRUCTIONS ............................................................................................................... 14
5. System Components ............................................................................................................. 18
5.1. UNPACKING THE DEVICE ............................................................................................................. 18
5.2. VENTILATOR – FRONT PANEL ....................................................................................................... 19
5.3. VENTILATOR – REAR PANEL......................................................................................................... 19
5.4. VENTILATOR – USE CONFIGURATION ............................................................................................. 2
5.5. PATIENT CIRCUIT ....................................................................................................................... 21
5.6. PNEUMATIC SECTION – THEORY OF OPERATION ................................................................................ 22
6. Connecting the Ventilator ..................................................................................................... 25
6.1. FRONT PANEL CONNECTIONS ....................................................................................................... 25
6.2. REAR PANEL CONNECTIONS......................................................................................................... 25
7. Power On and Display Startup Screen ................................................................................... 26
7.1. QUICK START ........................................................................................................................... 26
8. Navigating the GUI screens ................................................................................................... 28
8.1. SELECTING SCREEN OPTIONS ....................................................................................................... 28
8.2. EDITING FIELDS ........................................................................................................................ 28
8.3. NAVIGATING BETWEEN SCREENS .................................................................................................. 28
8.4. CONFIRMING OR CANCELLING A MESSAGE ...................................................................................... 29
8.5. SYMBOLS USED IN THE SYSTEM ..................................................................................................... 29
8.6. SYSTEM INDICATORS .................................................................................................................. 3
8.6.1. Battery Status .............................................................................................................. 30
8.6.2. indicators ..................................................................................................................... 31
8.6.3. lert ............................................................................................................................. 31
9. Getting Started with Ventway Sparrow ................................................................................. 32
9.1. DISCONNECT PATIENT ................................................................................................................ 32
9.2. PATIENT WEIGHT ...................................................................................................................... 33
9.3. VENTILATION MODE .................................................................................................................. 34
9.4. VENTILATION PARAMETERS ......................................................................................................... 35
9.5. NUMERICAL REPRESENTATION OF BREATH PARAMETERS .................................................................... 36
9.6. PRESSURE AND FLOW GRAPHS ...................................................................................................... 37
10. Main Menu ......................................................................................................................... 38

Ventway Sparrow User Manual
Page 5 of 11
1 .1. MAIN MENU/NEW PATIENT (DISCONNECT PATIENT SCREEN)........................................................... 39
1 .2. MAIN MENU/VENT PARAMS .................................................................................................... 39
10.2.1. M IN MENU/Vent Params/Trigger Sensitivity .......................................................... 40
10.2.2. M IN MENU/Vent Params/Vent Mode ..................................................................... 40
10.2.3. M IN MENU/Vent Params/O
2
Enrichment ................................................................ 40
10.2.4. M IN MENU/Vent Params/Patient Weight ............................................................... 40
1 .3. MAIN MENU/ALERT SETTINGS ................................................................................................ 41
1 .4. MAIN MENU/ADVANCED SETTINGS ............................................................................................ 42
10.4.1. M IN MENU/ dv Settings/Load Default ................................................................... 42
10.4.2. M IN MENU/ DV SETTINGS/Brightness ................................................................... 42
10.4.3. M IN MENU/ dv Settings/ lert volume ................................................................... 43
10.4.4. M IN MENU/ dv Settings/Language ........................................................................ 43
10.4.5. M IN MENU/ dv Settings/Tech mode ...................................................................... 43
1 .4.5.1. MAIN MENU/ADV SETTINGS/TECH MODE/Set Time ......................................... 44
1 .4.5.2. MAIN MENU/ADV SETTINGS/TECH MODE/Calibration ..................................... 44
1 .4.5.3. MAIN MENU/ADV SETTINGS/TECH MODE/Work Hours ................................... 45
1 .4.5.4. MAIN MENU/ADV SETTINGS/TECH MODE/Self Test ......................................... 46
1 .4.5.4.1. MAIN MENU/ADV SETTINGS/TECH MODE/Self Test /Flow and Pressure .. 47
1 .4.5.4.2. MAIN MENU/ADV SETTINGS/TECH MODE/Self Test /CVT ........................ 48
1 .4.5.4.3. MAIN MENU/ADV SETTINGS/TECH MODE/SELF TEST /VVT ...................... 49
1 .4.5.4.4. MAIN MENU/ADV SETTINGS/TECH MODE/SELF TEST /Blower Test ......... 5
1 .4.5.5. MAIN MENU/ADV SETTINGS/TECH MODE/Logbook ......................................... 51
1 .4.5.6. MAIN MENU/ADV SETTINGS/TECH MODE/Export Log ...................................... 51
1 .4.5.7. MAIN MENU/ADV SETTINGS/TECH MODE/SW Version .................................... 51
1 .4.5.8. MAIN MENU/ADV SETTINGS/TECH MODE/SW Update..................................... 52
1 .4.5.9. MAIN MENU/ADV SETTINGS/TECH MODE/System Mode ................................. 52
1 .4.5.1 . MAIN MENU/ADV SETTINGS/TECH MODE/Altitude ........................................ 52
11. Warnings and Alerts ............................................................................................................ 53
11.1. SUMMARY OF ALERT TYPES ....................................................................................................... 53
11.2. SUMMARY OF ALERT LEVELS ...................................................................................................... 54
11.3. WARNING EXAMPLE ................................................................................................................ 55
11.4. ALERT EXAMPLE ..................................................................................................................... 55
12. Default Parameters ............................................................................................................. 56
12.1. START VOLUME VENTILATION .................................................................................................... 56
12.1.1. Oxygen supply ............................................................................................................ 56
12.1.1.1. Connecting the Air Inlet to the Demand Valve .................................................. 57
12.1.2. Recommended devices for monitoring of oxygen ...................................................... 58
12.2. ALERT DEFAULT PARAMETERS: 5 KG TO 7 + KG ........................................................................... 59
12.3. ALERT DEFAULT PARAMETERS: ALL PATIENT WEIGHTS ..................................................................... 59
13. Labels and Symbols ............................................................................................................. 60
13.1. LABELS ................................................................................................................................. 6
13.2. SYMBOLS .............................................................................................................................. 61
14. Cleaning and Disinfecting .................................................................................................... 62
15. Ventilation Methods ........................................................................................................... 63
15.1. SIMV-VC (PS)FLOW CHART..................................................................................................... 63

Ventway Sparrow User Manual
Page 6 of 11
15.2. CPAP – SPONTANEOUS BREATHING MODE ................................................................................... 66
15.3. BACKUP VENTILATION MODE .................................................................................................... 69
15.3.1. Backup Ventilation in CP P ........................................................................................ 69
15.3.2. Backup Ventilation before starting patient ventilation .............................................. 70
15.4. SIMV VC (CPR) .................................................................................................................... 72
15.5. CPAP (CPR) ......................................................................................................................... 73
16. Service and Maintenance .................................................................................................... 74
16.1. DEVICE CALIBRATION AND SOFTWARE UPGRADES .......................................................................... 74
17. Troubleshooting .................................................................................................................. 75
18. Specifications ...................................................................................................................... 76
18.1. DIMENSIONS AND WEIGHT ....................................................................................................... 76
18.2. ENVIRONMENTAL SPECIFICATIONS .............................................................................................. 77
18.3. HARSH ENVIRONMENTAL CONDITIONS ............................................................................. 77
18.3.1. Operation in extreme high / low temperatures ......................................................... 77
18.3.2. Operation in High or Low ltitude ............................................................................. 78
18.3.3. irborne particulates ................................................................................................. 78
18.4. POWER SUPPLY ...................................................................................................................... 79
18.5. VENTILATION PERFORMANCE ..................................................................................................... 8
18.6. STANDARDS AND SAFETY REQUIREMENTS ..................................................................................... 81
19. Cleaning and outine Maintenance ..................................................................................... 82
20. Batteries ............................................................................................................................. 83
2 .1. BATTERY MAINTENANCE .......................................................................................................... 83
21. Parts and Accessories .......................................................................................................... 84
22. egulatory .......................................................................................................................... 85
23. Warranty ............................................................................................................................ 86
24. Appendix – Test Alerts ........................................................................................................ 87
24.1. BACKUP VENTILATION .............................................................................................................. 87
24.2. START BACKUP VENTILATION WITH UNKNOWN WEIGHT .................................................................... 88
24.3. CPAP VENTILATION WITH BACKUP VENTILATION ............................................................................ 89
24.4. PATIENT DISCONNECT .............................................................................................................. 9
24.5. HIGH PEEP ........................................................................................................................... 91
24.6. VALVE BLOCKED ...................................................................................................................... 92
24.7. PRESSURE ALERT ..................................................................................................................... 93
24.8. MINUTE VOLUME (MV) ALERT .................................................................................................. 94
24.9. LEAK ALERT ........................................................................................................................... 95
24.1 . TIDAL VOLUME ALERT ............................................................................................................ 96
24.11. I:E ALERT ............................................................................................................................ 97
24.12. APNEA ALERT ....................................................................................................................... 98
24.13. POWER ALERT ...................................................................................................................... 99
24.14. LOW BATTERY ALERT ........................................................................................................... 1
24.15. BATTERY TYPE ALERT ............................................................................................................ 1 1
24.16. VOLTAGE ALERT .................................................................................................................. 1 2
24.17. TEMPERATURE ALERT ........................................................................................................... 1 3
24.18. TUBE DISCONNECT ALERT ..................................................................................................... 1 4
24.19. SERVICE REQUIRED ALERT ..................................................................................................... 1 5
24.2 . FILTER REPLACEMENT REQUIRED ............................................................................................ 1 6

Ventway Sparrow User Manual
Page 7 of 11
24.21. ALTITUDE CHANGE ALERT ..................................................................................................... 1 7
24.22. SHUTDOWN ALERT .............................................................................................................. 1 8
24.23. VENTILATION DURING STANDBY (DUE TO PATIENT INSPIRATORY EFFORT) .......................................... 1 9
25. Appendix – Menu Hierarchy .............................................................................................. 110

Ventway Sparrow User Manual
Page 8 of 11
Obtaining Help
If you have a ventilator problem that you cannot solve, and the ventilator was
purchased directly from Inovytec, you may contact Inovytec at
If you have a ventilator problem that you cannot solve, and the ventilator was
purchased from an authorized Inovytec distributor, please contact your
distributor directly to report the problem.
Note: If this ventilator has not been purchased directly
from Inovytec, please ensure that it has been purchased
from an authorized distributor of Inovytec. To obtain a list
of authorized distributors, contact Inovytec at

Ventway Sparrow User Manual
Page 9 of 11
1. ABOUT THIS USER MANUAL
This User Manual provides the information necessary to operate and maintain
the Ventway Sparrow ventilator.
PLEASE EAD THIS USE MANUAL BEFO E OPE ATING THE SYSTEM. If any
part of this User Manual is not clear, contact Customer Support for assistance.
1.1. TYPES OF WARNINGS, CAUTIONS AND NOTES
Three types of special message appear in this User Manual:
Warning: A warning indicates precautions to avoid the
possibility of personal injury or death.
Caution: A caution indicates a condition that may lead
to damage to equipment, or a lower quality of treat-
ment.
Note: A note provides other important information.
PLEASE ETAIN THIS USE MANUAL FO FUTU E EFE ENCE.
Ventway Sparrow User Manual
Page 1 of 11
1.2. GLOSSARY AND ABBREVIATIONS
Apnea Temporary cessation of breathing
BPM Breaths Per Minute
CPAP Continuous Positive Airway Pressure
lpm Liters per minute
Mandatory
Breath
Ventilator initiated breath
MV Minute Volume
NIV Non-Invasive Ventilation
Peak Flow Maximum volumetric flow
PEEP Peak End Expiratory Pressure
PIP Peak Inspiratory Pressure
Pressure
support
Preset pressure delivered to the patient, on top of the
PEEP, during triggered breath
PSV Pressure Support Ventilation
SIMV VC
PS
Synchronized Intermittent Mechanical Ventilation with
Volume Control and Pressure Support
T
e
Expiratory Time
T
i
Inspiratory Time
Tidal
Volume
Normal volume of air displaced between normal
inhalation and exhalation when extra effort is not applied
Triggered
breath
Patient initiated breath
TV
e
Expired Tidal volume
TV
i
Inspired Tidal volume

Ventway Sparrow User Manual
Page 11 of 11
2. OVERVIEW OF SYSTEM
2.1. DESCRIPTION OF DEVICE
The Ventway is an emergency portable ventilator, used for transport, EMS and
military applications.
The situations for which it is intended are characterized by attendance of first
responders with limited triage capabilities, requiring a simple yet highly
effective ventilator, that is self-sufficient and lightweight.
The ventilator is suitable for noninvasive ventilation for a full non-vented
ventilation face mask or invasive ventilation via an endotracheal tube, tra-
cheostomy and laryngeal mask airway (LMA).
Note: The power supply needs to be firmly and permanently
secured in any EMS environment in which the ventilator is
used.

Ventway Sparrow User Manual
Page 12 of 11
3. CONDITIONS FOR USE
3.1. INTENDED USE
The Ventway Sparrow lung ventilator is intended for emergency use and
transportation of adult and pediatric patients weighing at least 5 kg (11 lb). It
may be used for invasive or noninvasive ventilation presets. The ventilator is
a restricted medical device intended for use by qualified, trained personnel
under the direction of a physician.
3.2. INDICATIONS FOR USE
The Ventway Sparrow ventilator is intended to provide continuous or
intermittent ventilatory support for the care of individuals who require
mechanical ventilation. Specifically, the ventilator is applicable for adult and
pediatric patients weighing at least 5 kg, who require the following types of
ventilatory support: SIMV - VC (PS), CPAP.

Ventway Sparrow User Manual
Page 13 of 11
3.3. CONTRAINDICATIONS
See specific patient instructions regarding performing ventilation or any
use of life support equipment.
Acute Pneumothorax
3.4. LIMITATIONS OF USE
Clinical situations potentially affecting accuracy or performance:
Controlling the flow in the presence of difficult airways, such as severe
lung blockage and asymmetric air entrance to the lung
Low compliance of the airways
Asynchronization between patient and ventilator
Barotrauma
Behavior of the ventilator in case of barotrauma, monitoring and alerting
in these cases.
Note: The use of humidification is not recommended, due to
potential blockage of control and measurement tubes.

Ventway Sparrow User Manual
Page 14 of 11
4. SAFETY
4.1. ELECTRICAL SAFETY
The device complies with requirements of IEC/EN 6 6 1-1 for general
requirements for safety of medical electrical equipment:
Class I Equipment BF type applied part
Mode of operation: Continuous measurement
Degree of mobility: Portable
4.2. EMC COMPLIANCE
The unit has Class B compliance.
4.3. SAFETY INSTRUCTIONS
Warnings
Basic safety precautions always should be taken, including all those
listed below.
DO NOT USE BEFORE READING THIS USER MANUAL.
DO NOT use this device for any purpose other than specified in this
manual without written consent and approval from Inovytec Medical
Solutions Ltd.
In case of VENTILATOR failure, the lack of immediate access to
appropriate alternative means of ventilation can result in PATIENT
death.
US Federal Law restricts the sale of this instrument only by, or on the
order of, a physician.
The exhaled volume of the patient can differ from the measured
exhaled volume due to leaks around the mask.
The device shall not be used in a hyperbaric chamber.
The device shall not be used with nitric oxide and explosive or highly
flammable gas mixtures.
The device shall not be used with helium or mixtures with helium.
The device should not be used on unattended patients.
The device accuracy can be affected by the gas added by use of a
nebulizer.

Ventway Sparrow User Manual
Page 15 of 11
Cautions
If the device packaging is not intact, do not use the device.
If the device does not turn on, or is not working correctly, discontinue
use. Refer servicing or replacement to qualified service personnel.
Do not disassemble any part of the system components. This system
is not user-serviceable.
Do not use the equipment if it is not working properly or if it has
suffered any damage, for example, by dropping the equipment or
splashing water on it.
If the LCD screen is cracked or damaged, check whether the screen
can be used, and if not, do not use the device.
If the power button is damaged or stuck, disconnect the patient from
the device and remove the battery.
If the rotator switch does not allow changing parameters, the device
cannot be used.
The Patient Circuit is single use only. If it is not removed from a new
container, it may have already been used and should not be used.
The Patient Circuit can be used for the same patient up to five days.
Do not use a Patient Circuit that is not the original Patient Circuit of
the device.
Confirm that the expiration date, found on the Patient Circuit
packaging bag, has not been reached.
The device should be used under medical supervision.
When using external oxygen enrichment, please note the following:
When using demand valve, the oxygen enrichment level will
reach a minimum of 95%.
When using reservoir bag, the oxygen enrichment level may
vary depending on oxygen flow rate.
It is the user’s responsibility to retain information about the patient
(by USB connection). Storage capacity may be sufficient for at least
one year of ventilation.

Ventway Sparrow User Manual
Page 16 of 11
Cautions
Repairs should be undertaken only by personnel trained or auth-
orized by Inovytec Medical Solutions Ltd. Do not modify this equip-
ment without authorization from Inovytec Medical Solutions Ltd.
The device may not operate correctly if used or stored outside the
relevant temperature or humidity ranges, as described in the per-
formance specifications.
Strictly follow the warning instructions in this manual.
This instrument is fragile. To prevent damage, please handle with
care, including while packing and unpacking.
Ensure that the system is only used by a trained person familiar with
all system operating procedures. EMS personnel should complete a
training program before operating the Ventway Sparrow.
User is prohibited from changing, adding, removing or disassembling
any system parts. Warranty shall not apply to any defects, failure or
damage caused by improper use and/or improper or inadequate
maintenance and care.
The unit is classified as Class IIb, continuously operated, ordinary
equipment with applied part and with signal input/output parts. The
device is not intended for use in the presence of flammable sub-
stances.
To avoid damage to the screen, do not expose the instrument to
direct sunlight for prolonged periods.
The system is approved for IP45 in operation mode with oxygen
enrichment. To prevent damage to the instrument or patient cable,
avoid liquid spillage while cleaning.
It is strongly recommended that all Ventway Sparrow parts be
replaced with parts purchased from Inovytec Medical Solutions Ltd.
or an authorized distributor. Use of other parts may damage the unit
and void the warranty.
The ventilator is suitable for noninvasive ventilation for full non-
vented ventilation face mask or invasive ventilation via an
endotracheal tube, tracheostomy and laryngeal mask airway (LMA).

Ventway Sparrow User Manual
Page 17 of 11
Cautions
During NIV (Non-Invasive Ventilation) the user should use a
capnograph in order to monitor the CO2 level of the patient.
Covering the ventilator is prohibited.
Ensure that no Latex or natural rubber parts are in patient pathways.
When adding medication to the gas flowing into the patient by using
an MDI or nebulizer, please position between mask/ETT and exha-
lation valve.
Do not obstruct the gas intake ports.
Discarded used or unused patient circuit is classified as clinical waste.
As such, the user is responsible for complying with all local and
national regulations regarding discarding of clinical waste.
Notes
Dispose of this device and used sensors in accordance with
local regulations.
Use the equipment only for the purpose described in these
instructions for use.
The contents of this manual are subject to change without prior
notice.
The user or any technical personnel who are not formally
authorized by Inovytec Medical Solutions Ltd. should not open
the device under any circumstances. Opening the device could
damage the unit and will void the warranty provided by
Inovytec Medical Solutions Ltd.

Ventway Sparrow User Manual
Page 18 of 11
5. SYSTEM COMPONENTS
5.1. UNPACKING THE DEVICE
Package contents
1 Ventway Sparrow ventilator
2 User Manual and device documentation
3 Battery pack - Inside ventilator battery
compartment
4 Power supply
Ventway Sparrow User Manual
Page 19 of 11
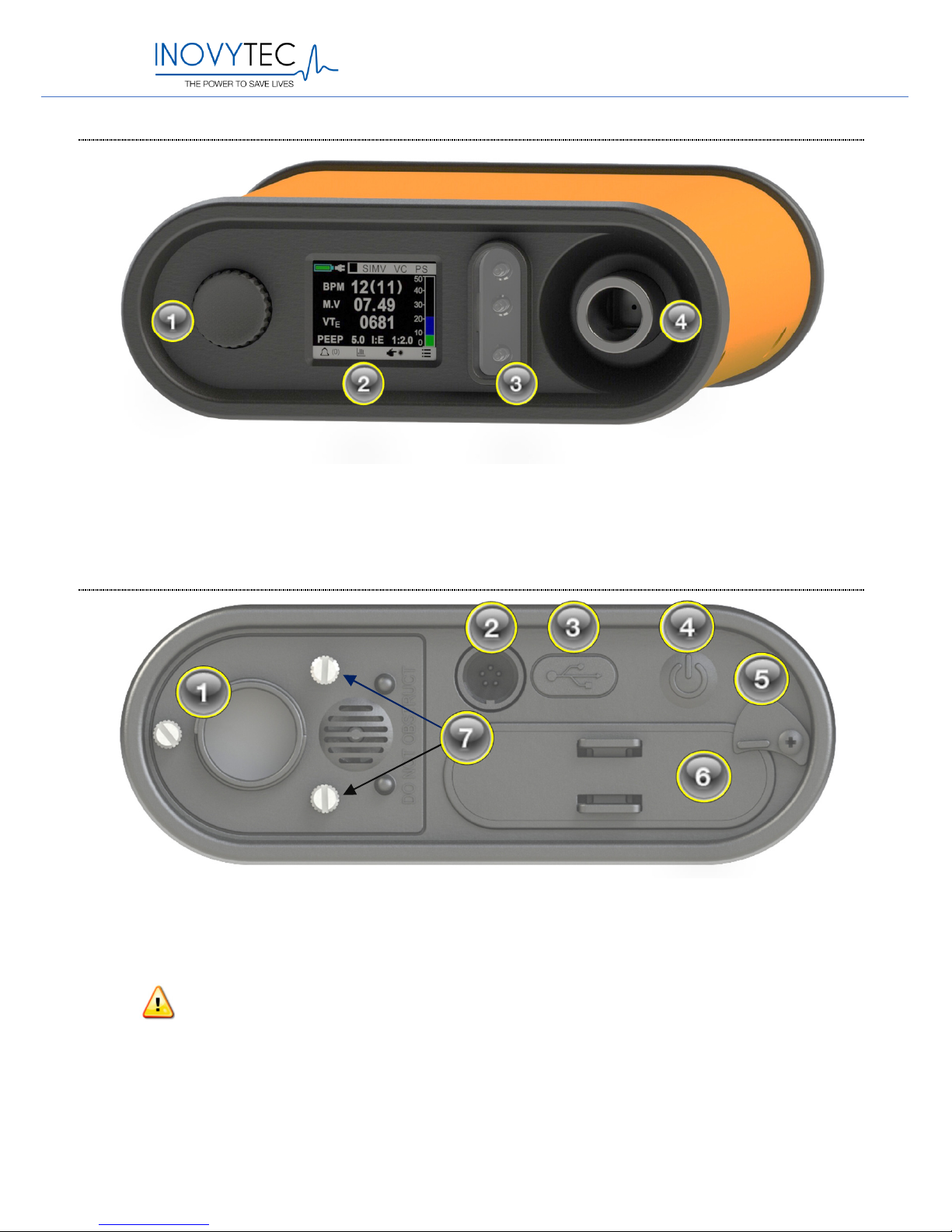
5.2. VENTILATOR – FRONT PANEL
5.3. VENTILATOR – REAR PANEL
Warning: Do not block item (1), the air/oxygen inlet.
Rear Pane:
(1)
ir/Lo
w pressure oxygen inlet,
(2)
P
ower supply connector,
(3)
USB
connector, (4) Power On/Off button, (5) Battery pack lock, (6) Battery pack,
(7) Filter compartment screws
Front Panel:
(1)
control knob,
(2)
display,
(3)
control and sensing tubes port,
(4) patient port

Ventway Sparrow User Manual
Page 2 of 11
5.4. VENTILATOR – USE CONFIGURATION
During transport, the ventilator is recommended to be placed in a horizontal
position.
Other manuals for Ventway Sparrow
1
This manual suits for next models
2
Table of contents
Other Inovytec Medical Equipment manuals
Popular Medical Equipment manuals by other brands
Pressalit
Pressalit R2011 Mounting instruction

Mindray
Mindray accutorr plus operating instructions

Mindray
Mindray Resona I9W Operator's manual
Nasco Healthcare
Nasco Healthcare C.H.A.R.L.I.E. LF01420U manual
ORTHOSERVICE RO+TEN
ORTHOSERVICE RO+TEN polfit 17 user manual
Mizuho
Mizuho Seat Extension Instructions for use