Teleflex LMA Unique Cuff Pilot User manual

Page 1of 5
EN – English
INSTRUCTIONS FOR USE –
LMA
Unique™ (S) & LMA
Unique™ (S)
Cuff Pilot™
CAUTION: Federal (USA) law restricts this device to sale by or on the
order of a physician.
WARNING: LMA
Unique™ (S) and LMA
Unique™ (S) Cuff Pilot™are supplied
sterile for single use only, should be used straight from the pack and should be
discarded after use. They must not be re-used. Reuse may cause cross infection
and reduce product reliability and functionality.
WARNING: Re-processing of LMA
Unique™ (S) and LMA
Unique™ (S) Cuff
Pilot™ intended for single use only may result in degraded performance or loss
of functionality. Re-use of single use only products may result in exposure to
viral, bacterial, fungal, or prionic pathogens. Validated cleaning and sterilisation
methods and instructions for reprocessing to original specifications are not
available for these products. LMA
Unique™ (S) and LMA
Unique™ (S) Cuff
Pilot™ are not designed to be cleaned, disinfected, or re-sterilised.
GENERAL INFORMATION:
Unless otherwise stated, the reference to “device” stated on this IFU applies to
both versions of LMAUnique™ (S) & LMAUnique™ (S) Cuff Pilot™.
The devices are only for use by medical professionals trained in airway
management.
DEVICE DESCRIPTION:
Both LMAUnique™ (S) and LMAUnique™ (S) Cuff Pilot™ are made primarily of
silicone and are supplied sterile (sterilised by Ethylene Oxide) for single use only.
The devices are not made with natural rubber latex and phthalates.
LMAUnique™ (S) and LMAUnique™ (S) Cuff Pilot™ have three main
components: airway tube, cuff and inflation system.
The inflation system of LMAUnique™ (S) consists of an Inflation Line with Pilot
Balloon and Check Valve for cuff inflation and deflation. The Pilot Balloon provides
an indication of the pressure within the cuff and the Check Valve prevents leakage
of air and maintains the pressure in cuff.
The inflation system of LMAUnique™ (S) Cuff Pilot™ consists of an Inflation Line
with Cuff Pilot™ technology. Cuff Pilot™ technology enables constant visualisation
of inside mask cuff pressure. It replaces the standard pilot balloon and to be used
in the same way for cuff inflation and deflation.
LMAUnique™ (S) is MR conditional. Refer to MRI information section prior using
the device in MRI environment.
LMAUnique™ (S) Cuff Pilot™ is MR Safe. The term ‘MR Safe’ means that it poses
no known hazards in all MR environments.
Figure 1: LMAUnique™ (S) components
LMAUnique™ (S) components (Figure 1):
a) Connector
b) Airway Tube
c) Backplate
d) Cuff
e) Inflation Line
f) Pilot Balloon
g) Check Valve
Figure 2: LMAUnique™ (S) Cuff Pilot™ components
LMAUnique™ (S) Cuff Pilot™ components (Figure 2):
a) Connector
b) Airway Tube
c) Backplate
d) Cuff
e) Inflation Line
f) Cuff Pilot™
Page 2of 5
Table 1: Specification for the device
Device Size
1
1.5
2
2.5
3
4
5
6
Patient
Weight
(kg)
Up
to 5 5-10 10-
20
20-
30
30-
50
50-
70
70-
100 >100
Airway
Connector
15 mm male (ISO 5356-1)
Inflation
Valve Luer cone (ISO 594-1)
Internal
Volume of
ventilator
pathway
(ml)
4 5 7 11 18 18 25 28
Pressure
drop
(cm H2O
at l/min)
< 2.7
cm
H2O
at 15
l/min
< 1.1
cm
H2O
at 15
l/min
< 2.1
cm
H2O
at 30
l/min
< 1.0
cm
H2O
at 30
l/min
< 1.6
cm
H2O
at 60
l/min
< 1.6
cm
H2O
at 60
l/min
< 1.0
cm
H2O
at 60
l/min
< 1.0
cm
H2O
at 60
l/min
Min.
interdental
gap (mm)
16 18 21 24 25 30 34 38
Normal
length of
the
internal
ventilatory
pathway
(cm)
10.5 12.0 13.8 15.0 19.5 19.5 21.3 21.8
A summary of the methods, materials, data and results of clinical studies that
validate the requirements of this international standard is available on request, if
applicable.
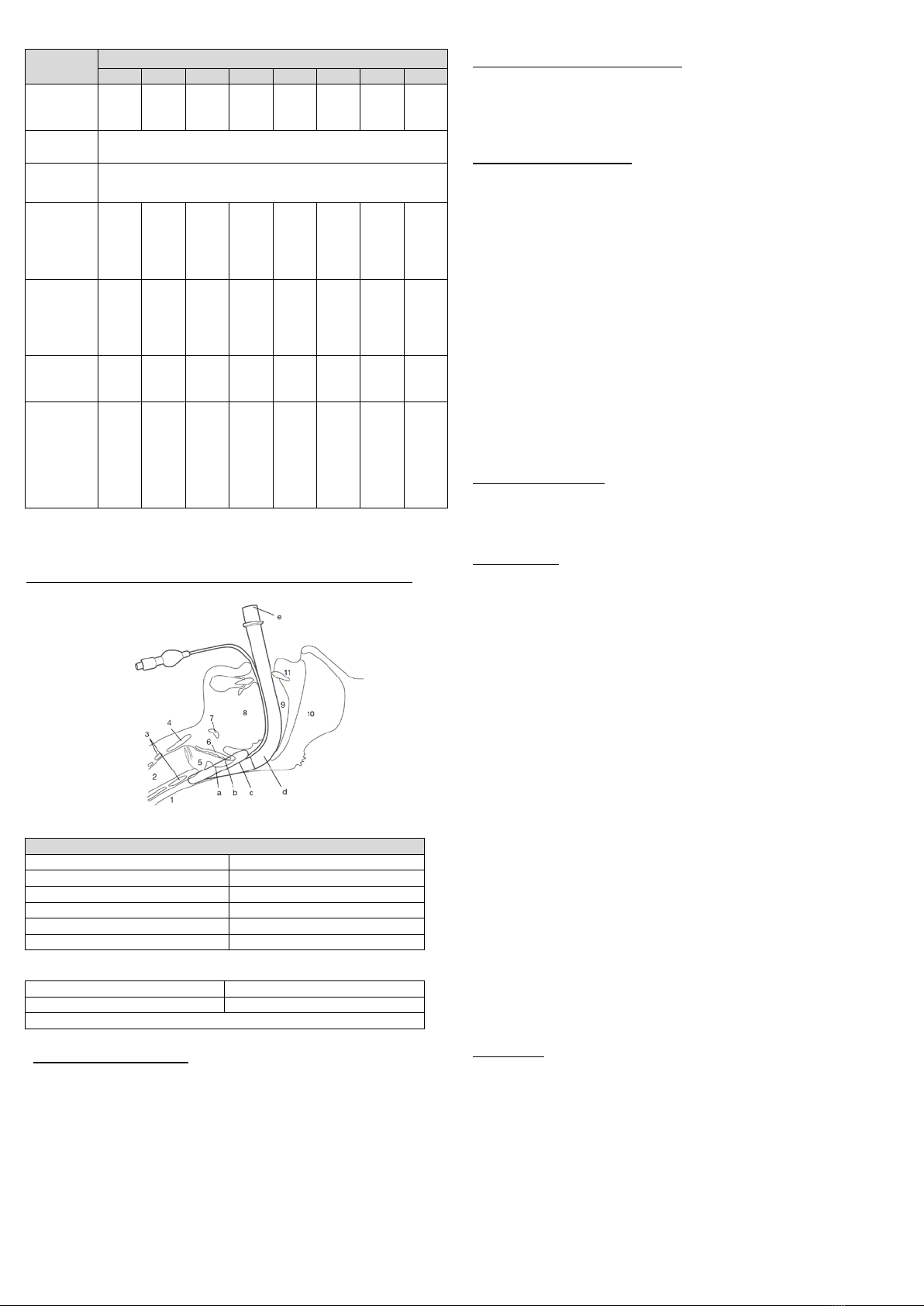
Figure 3: Correct Position of the device in relation to anatomical landmarks
Table 2: Description of anatomical landmarks
Anatomical Landmarks
1 - Esophagus
7 - Hyoid bone
2 - Trachea
8 - Tongue
3 - Cricoid cartilage
9 - Buccal cavity
4 - Thyroid cartilage
10 - Nasopharynx
5 - Laryngeal inlet
11 - Incisors
6 - Epiglottis
Table 3: Description of the device parts
a - Patient end
d - Ventilatory pathway
b - Ventilatory opening
e - External end connector
c - Sealing mechanism
INDICATION FOR USE:
LMAUnique™ (S) and LMAUnique™ (S) Cuff Pilot™ are indicated for use in
achieving and maintaining control of the airway during routine and emergency
anaesthetic procedures in fasted patients using either spontaneous or Positive
Pressure Ventilation (PPV).
They are also indicated for securing the immediate airway in known or unexpected
difficult airway situations.
They are best suited for use in elective surgical procedures where tracheal
intubation is not necessary.
They may be used to establish an immediate, clear airway during cardiopulmonary
resuscitation (CPR) in the profoundly unconscious patient with absent
glossopharyngeal and laryngeal reflexes requiring artificial ventilation. In these
cases, the devices should be used only when tracheal intubation is not possible.
RISK-BENEFIT INFORMATION:
When used in the profoundly unresponsive patient in need of resuscitation or in a
difficult airway patient on an emergency pathway (i.e., “cannot intubate, cannot
ventilate”), the risk of regurgitation and aspiration must be weighed against the
potential benefit of establishing an airway.
CONTRAINDICATIONS:
Due to the potential risk of regurgitation and aspiration, do not use LMA®
Unique™ (S) and LMA® Unique™ (S) Cuff Pilot™ as a substitute for an endotracheal
tube in the following elective or difficult airway patients on a non-emergency
pathway:
1. Patients who have not fasted, including patients whose fasting cannot be
confirmed.
2. Patients who are grossly or morbidly obese, more than 14 weeks pregnant or
emergency and resuscitation situations or any condition associated with delayed
gastric emptying, or using opiate medication prior to fasting.
The device is also contraindicated in:
3. Patients with fixed decreased pulmonary compliance, or peak insufflation
pressure anticipated to exceed 20 cm H2O, because the device forms a low-
pressure seal (approximately 20 cm H2O) around the larynx.
4. Adult patients who are unable to understand instructions or cannot adequately
answer questions regarding their medical history, since such patients may be
contraindicated for the device.
5. The device should not be used in the resuscitation or emergency situation in
patients who are not profoundly unconscious and who may resist device insertion.
ADVERSE EFFECTS:
There are reported adverse reactions associated with the use of laryngeal mask
airways. Standard textbooks and published literature should be consulted for
specific information.
WARNINGS:
1. To avoid trauma, excessive force must be avoided at all times.
2. Do not use if the device is damaged or its unit packaging is damaged or opened.
3. When using the device in special environmental conditions, such as enriched
oxygen, ensure that all necessary preparation and precautions have been taken,
especially with regard to fire hazards and prevention. The device may be
flammable in the presence of lasers and electrocautery equipment.
4. It is most important that pre-use checks are carried out on the device prior to
use, in order to establish whether it is safe for use. Failure of any one test
indicates the device should not be used.
5. Do not immerse or soak the device in liquid prior to use.
6. When applying lubricant avoid blockage of the airway aperture with the
lubricant.
7. After the pre-use checks are complete never overinflate the cuff over 60cm H2O.
Excessive intra-cuff pressure can result in malposition and pharyngo-laryngeal
morbidity, including sore throat, dysphagia and nerve injury.
8. A water-soluble lubricant, such as K-Y Jelly®, should be used. Do not use
silicone-based lubricants as they degrade the device components. Lubricants
containing Lidocaine are not recommended for use with the device. Lidocaine can
delay the return of the patient’s protective reflexes expected prior to removal of
the device, may possibly provoke an allergic reaction, or may affect the
surrounding structures, including the vocal cords.
9. The device does not prevent regurgitation or aspiration. Its use in anaesthetised
patients should be restricted to fasting patients. A number of conditions
predispose to regurgitation under anaesthesia. Do not use the devices without
taking appropriate precautions to ensure the stomach is empty.
10. Diffusion of nitrous oxide, oxygen, or air may increase or decrease cuff volume
and pressure. In order to ensure that cuff pressures do not become excessive, cuff
pressure should be measured regularly during a case with a cuff pressure monitor.
11.Refer to MRI information section prior to using the devices in MRI
environment.
CAUTIONS:
1. Laryngeal spasm may occur if the patient becomes too lightly anaesthetized
during surgical stimulation or if bronchial secretions irritate the vocal cords during
emergence from anaesthesia. If laryngeal spasm occurs, treat the cause. Only
remove the device when airway protective reflexes are fully competent.
2. Do not pull or use undue force when handling the inflation line or try to remove
the device from patient by the inflation line as it may detach from the cuff spigot.
3. Only use a syringe with standard luer taper tip for inflation or deflation.
4. Only use with the recommended manoeuvres described in the instructions for
use.
5. If airway problems persist or ventilation is inadequate, the device should be
removed and an airway established by some other means.
Page 3of 5
6. Careful handling is essential. Avoid contact with sharp or pointed objects at all
times to prevent tearing or perforation of the device. Do not insert the device
unless the cuffs are fully deflated as described in the instructions for insertion.
7. Gloves should be worn during preparation and insertion to minimize
contamination of the airway.
8. Used device shall follow a handling and elimination process for bio-hazard
products, in accordance with all local and national regulations.
9. Store the device in a dark cool environment, avoiding direct sunlight or
extremes of temperatures.
10. Ensure all removable denture work is removed before inserting the device.
11. An unreliable or obstructed airway may result in cases where the device has
been incorrectly inserted.
PREPARATION FOR USE:
Choose the correct size of device. Refer to Table 1 for patient weight and size
information.
Keep a clearly marked syringe for inflation and deflation of the cuff.
PRE-USE CHECKS:
Warning: It is most important that pre-use checks are carried out on the device
prior to use, in order to establish whether it is safe for use.
Warning: Failure of any one test indicates the device should not be used.
These tests should be carried out as follows:
1. Examine the interior of the airway tube to ensure it is free from blockage or
loose particles. Examine the tube throughout its length. Should any cuts or
indentations be found, discard the device.
2. Holding at each end flex the airway tube to increase its curvature up to but not
beyond 180o. Should the tube kink during this procedure, discard the device.
3. Deflate the cuff fully.
For LMA
Unique™ (S)
Re-inflate the device with a volume of air 50% greater than the maximum inflation
value for each size.
Table 4: Test cuff over-inflation volumes
Device Size
1
1.5
2
2.5
3
4
5
6
Over-
inflation
cuff
volumes
(ml)
6 10 15 21 30 45 60 75
Examine the cuff for leaks, herniations and uneven bulging. If any indications of
these problems exist, discard the device. A herniating mask may cause obstruction
during use.
While the device remains 50% over-inflated, examine the inflation pilot balloon.
The balloon shape should be elliptical, not spherical. Then, deflate the mask again.
For LMA
Unique™ (S) Cuff Pilot™
Re-inflate the device to Red Zone of Cuff Pilot™ (Fig 14) with a volume of air >
70cmH20.
Examine the cuff for leaks, herniations and uneven bulging. If any indications of
these problems exist, discard the device. A herniating mask may cause obstruction
during use. Then, deflate the mask again.
4. Examine the airway connector. It should fit securely into the airway tube and it
should not be possible using reasonable force, to remove. Do not use excessive
force or twist the connector as this may break the seal. If the connector is loose,
discard the device to avoid the risk of accidental disconnection during use.
5. Discoloration. Discoloration affects visibility of fluid in the airway tube.
6. Gently pull the inflation line to ensure it is securely attached to both the cuff
and balloon.
7. Examine the aperture in the mask. Gently probe the two flexible bars
traversing the mask aperture to ensure they are not broken or otherwise damaged.
If the aperture bars are not intact, the epiglottis may obstruct the airway. Do not
use if the aperture bar is damaged.
PRE-INSERTION PREPARATION:
Deflate the cuff completely in order to create the stiff thin leading edge necessary
to wedge the tip behind the cricoid cartilage. The cuff should fold back away from
the aperture bars. Lubricate the back of the cuff thoroughly just before insertion.
Do not lubricate the front as this may result in blockage of aperture bar or
aspiration of lubricant.
Warning: A water-soluble lubricant, such as K-Y Jelly®, should be used. Do not use
silicone-based lubricants as they degrade the device components. Lubricants
containing Lidocaine are not recommended for use with the device. Lidocaine can
delay the return of the patient’s protective reflexes expected prior to removal of
the device, may possibly provoke an allergic reaction, or may affect the
surrounding structures, including the vocal cords.
Caution: Ensure all removable denture work is removed before inserting the
device.
INSERTION:
Caution: Gloves should be worn during preparation and insertion to minimize
contamination of the airway.
Caution: The patency of this device should be reconfirmed after any change in the
patient’s head or neck position.
Standard Insertion Method:
1. Anaesthesia must be deep enough to permit insertion.
Do not try to insert immediately following barbiturate induction, unless a relaxant
drug has been given.
2. Position the head and neck as for normal tracheal intubation.
Keep the neck flexed and the head extended by pushing the head from behind
with one hand while inserting the mask into the mouth with the other hand (Fig.4).
3. When inserting the mask, hold it like a pen with the index finger placed
anteriorly at the junction of the cuff and tube (Fig.4). Press the tip up against the
hard palate and verify it lies flat against the palate and that the tip is not folded
over, before pushing further into the pharynx.
4. Using the index finger, push the mask backwards still maintaining pressure
against the palate (Fig.5).
5. As the mask moves downwards, the index finger maintains pressure backwards
against the posterior pharyngeal wall to avoid collision with the epiglottis. Insert
the index finger fully into the mouth to complete insertion (Fig.6). Keep other
fingers out of the mouth. As insertion progresses, the flexor surface of the whole
index finger should lie along the tube, keeping it firmly in contact with the palate.
(Fig.6).
AVOID INSERTING WITH SEVERAL MOVEMENTS OR JERKING UP AND DOWN IN
THE PHARYNX AFTER RESISTANCE IS FELT.
When resistance is felt the finger should already have been fully inserted into the
mouth. Use the other hand to hold the tube while withdrawing the finger from the
mouth (Fig.7).
6. Check that the black dotted line on the tube faces the upper lip.
Now immediately inflate the cuff without holding the tube.
Do this BEFORE connection to the gas supply. This will permit the device to
position itself correctly. Inflate the cuff with sufficient air to obtain a low pressure
seal. Refer to Table 5 for inflation information. During cuff inflation, do not hold
the tube as this prevents the device from settling into its correct location.
Warning: NEVER OVERINFLATE THE CUFF.
Table 5: Inflation Information
Product Recommended
Device Size
1
1.5
2
2.5
3
4
5
6
LMA
Unique™
(S)
Maximum Cuff
inflation
volume
(ml/60cmH20)
4 7 10 14 20 30 40 50
LMA
Uniq
ue™ (S)
Cuff Pilot™
Intracuff
pressure
(cmH2O)
60 60 60 60 60 60 60 60
7. Connect to the gas supply, holding the tube, to prevent displacement. Gently
inflate the lungs to confirm correct placement. Insert a roll of gauze as a bite-block
(ensuring adequate thickness), and tape the device into place, ensuring that the
proximal end of the airway tube is pointing caudally. When correctly placed, the
tube should be pressed back into the palate and posterior pharyngeal wall. When
using the device, it is important to remember to insert a bite block at the end of
the insertion procedure.
Figure 4 Figure 5
Figure 6 Figure 7

Page 4of 5
Thumb Insertion Method:
This technique is suitable for patients in whom access to the head from behind is
difficult or impossible and during cardiopulmonary resuscitation. The device is
held with the thumb in the position occupied by the index finger in the standard
technique (Fig.8).The tip of the mask is pressed against the front teeth and the
mask is pressed posteriorly along the palate with the thumb. As the thumb nears
the mouth, the fingers are stretched forward over the patient’s face (Fig.9).
Advance the thumb to its fullest extent (Fig.10). The pushing action of the thumb
against the hard palate also serves to press the head into extension. Neck flexion
may be maintained with a head support. Before removing the thumb, push the
tube into its final position using the other hand (Fig.11).
Figure 8 Figure 9
Figure 10 Figure 11
Warnings:
- DO not use a Guedel (oropharyngeal) airway as a bite block, as it prevents
correct positioning of the device increasing trauma and reducing seal effectiveness.
- Once correctly positioned, the device must be securely taped in position to the
patient’s face to prevent its movement during use and loss of the patients’ airway.
- Do not move the patient or reposition the device during anaesthesia/surgery to
prevent stimulation of the airway that this may cause.
- The anaesthetic breathing system must be adequately supported once
connected to the device to avoid rotation of the mask and to ensure the tube is
bent only downwards on to the chin and never upwards to avoid loss of the
patient’s airway due to displacement.
- Ensure anaesthesia is adequate for the level of surgical stimulus to avoid gagging,
coughing and laryngospasm leading to displacement of the device.
Inflation System of LMA
Unique™ (S) Cuff Pilot™:
1. LMAUnique™ (S) Cuff Pilot™ has a cuff pilot valve, which enables the end user
to monitor the intracuff pressure of the mask through visual means while it is
inserted in the patient’s airway. There are three pressure zones on the Cuff Pilot
Valve – Yellow, Green and Red. The position of the black line on the bellows
indicates the pressure within the cuff.
2. The Green Zone designates optimal pressure of the cuff, between 40 - 60
cmH20. Air is introduced into the cuff until the black line is within this zone and a
seal has been obtained.
Figure 12: Cuff PilotValve in Green Zone
3. The Yellow Zone indicates a pressure of less than 40cmH20. A seal may be
obtained in the Yellow Zone; however, movement of the black line on the bellows
into the Yellow Zone during the procedure may indicate a possible decrease in
pressure or under-inflation.
Figure 13: Cuff PilotValve in Yellow Zone
4. The Red Zone indicates a pressure of more than 70cmH20. This indicates a
possible increase in pressure or over-inflation. It is recommended that the
pressure be released until the black bellows line is back in the Green Zone.
Figure 14: Cuff PilotValve in Red Zone
Warning: NEVER OVERINFLATE THE CUFF.
MAINTAINING THE AIRWAY:
1. Obstruction can occur if the device becomes dislodged or is incorrectly inserted.
The epiglottis may be pushed down with poor insertion technique. Check by
auscultation of the neck and correct by re-insertion or elevation of the epiglottis
using a laryngoscope.
2. Malposition of mask tip into the glottis may mimic bronchospasm.
3. Avoid moving the device about in the pharynx when the patient is at a light
plane of anaesthesia.
4. Keep the bite-block in place until the device is removed.
5. Do not deflate the cuff until reflexes have fully returned.
6. Air may be withdrawn from the cuff during anaesthesia to maintain a constant
intracuff pressure (always less than 60cm H2O).
REMOVAL:
1. The device, together with the recommended bite-block, should be left in place
until the return of consciousness. Oxygen should be administered using a “T”
piece system and standard monitoring should be in place. Before attempting to
remove or deflate the device, it is essential to leave the patient completely
undisturbed until protective reflexes have fully returned. Do not remove the
device until the patient can open the mouth on command.
2. Look for the onset of swallowing which indicates reflexes are almost restored. It
is usually unnecessary to perform suction because the correctly used device
protects the larynx from oral secretions. Patients will swallow secretions on
removal. Suction equipment should however be available at all times.
3. Deflate the cuff completely just prior to removal, although partial deflation can
be recommended in order to assist in the removal of secretions.
USE WITH MAGNETIC RESONANCE IMAGING (MRI):
For LMA
Unique™ (S)
LMAUnique™ (S) is MR Conditional.
Non-clinical testing demonstrated that this product is MR Conditional. A patient
with LMAUnique™ (S) can be scanned safely immediately after placement under
the following conditions:
•Before the patient enters the MRI system room, the airway must be fixed
properly in place with adhesive tape, cloth tape or other appropriate means
to prevent movement or dislodgement.
•Static magnetic field of 3-Tesla or less
•Maximum spatial gradient magnetic field of 720-Gauss/cm or less
•Maximum MR system reported, whole body averaged specific absorption rate
(SAR) of 4-W/kg (First Level Controlled Mode of operation for the MR system)
for 15 minutes of scanning (i.e., per pulse sequence)
MRI-Related Heating
Under the scan conditions defined above, LMAUnique™ (S) is expected to
produce a maximum temperature rise of 2.5°C after 15 minutes of continuous
scanning.
Artifact Information
The maximum artifact size as seen on a gradient echo pulse sequence and a 3 -
Tesla MR System extends approximately 50 mm relative to the size and shape of
LMAUnique™ (S).
For LMA
Unique™ (S) Cuff Pilot™
MR Safe
LMAUnique™ (S) Cuff Pilot™ is MR Safe (i.e., an item that poses no known
hazards in all MR environments).

Page 5of 5
SYMBOL DEFINITION:
Manufacturer
Consult IFU on this website: www.LMACO.com
Air inflation volume/Intra-cuff pressure
Patient weight
Caution (Read Instructions before use)
Not made with natural rubber latex
Fragile, handle with care
Keep away from sunlight
Keep dry
This way up
Product Code
Lot Number
CE Mark
Date of Manufacture
Do not re-use
Do not re-sterilise
This product not made with phthalates
Sterilised by Ethylene Oxide
Use By
Do not use if package is damaged
MR Conditional
MR Safe
Quantity
Copyright © 2017 Teleflex Incorporated.
All rights reserved. No part of this publication may be reproduced, stored in a
retrieval system or transmitted in any form or by any means electrical, mechanical,
photocopying, recording or otherwise, without the prior permission of the
publisher.
Teleflex, the Teleflex logo, LMA, LMA Unique and Cuff Pilot are trademarks or
registered trademarks of Teleflex Incorporated or its affiliates, in the US and/or
other countries.
LMAUnique™ (S) Cuff Pilot™ is protected by patents and patent applications of
Teleflex Incorporated or its affiliates in the US and/or other countries.
See www.lmaco.com/IP for details.
The information given in this document is correct at the time of going to press.
The manufacturer reserves the right to improve or modify the products without
prior notification.
Always consult the instructions on indications, contraindications, warnings and
precautions, or information on which LMAAirways are best suited for different
clinical applications.
Manufacturer’s Warranty:
LMAUnique™ (S) and LMAUnique™ (S) Cuff Pilot™ are designed for single use
and warranted against manufacturing defects at the time of delivery.
Warranty is applicable only if purchased from an authorized distributor. TELEFLEX
MEDICAL DISCLAIMS ALL OTHER WARRANTIES, WHETHER EXPRESS OR IMPLIED,
INCLUDING, WITHOUT LIMITATION, THE WARRANTIES OF MERCHANTABILITY OR
FITNESS FOR APARTICULAR PURPOSE.
Teleflex Medical
IDA Business and Technology Park
Dublin Road, Athlone, Co Westmeath, Ireland
Contact Information in USA:
Teleflex Medical
2917 Weck Drive, Research Triangle Park, NC 27709 USA
International: (919)544-8000
USA: (866) 246-6990
www.LMACO.com
Issue: PBT-2100-000 Rev AUK
Date of Issue: 2017-07
This manual suits for next models
3
Table of contents
Other Teleflex Medical Equipment manuals

Teleflex
Teleflex LMA Supreme User manual

Teleflex
Teleflex Arrow PICC User manual

Teleflex
Teleflex Arrow EZ-IO User manual

Teleflex
Teleflex LMA Unique PreCurved User manual

Teleflex
Teleflex ARROW GlideThru User manual

Teleflex
Teleflex LMA Supreme User manual

Teleflex
Teleflex Arrow EZ-IO User manual
Teleflex
Teleflex WECK EFx Shield User manual
Popular Medical Equipment manuals by other brands
C-me
C-me BodyGuard 575 Color Vision Operator's manual
Hillrom
Hillrom Liko AmputeeSling 70 Instruction guide

Etac
Etac immedia SlingOn Instructions for use

Care Fusion
Care Fusion GreenLight 4559GSP Instructions for use

Hillrom
Hillrom Yellofins Apex Instructions for use

B. Braun
B. Braun Easypump II ST Series Instructions for use