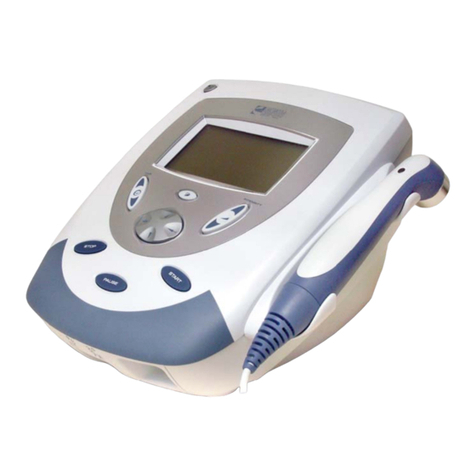
Intelect Legend 2 User manual

EN
Intelect®Legend 2
2 CHANNEL COMBO (12-5010), 4 CHANNEL COMBO (12-5011)
User Manual

2CONTENTS EN
INTELECT®LEGEND 2 USER MANUAL
FOREWORD 3
INTENDED PURPOSE 3
INTENDED USER 3
INTENDED ENVIRONMENT FOR USE 3
INTENDED PATIENT POPULATION 3
ELECTROTHERAPY INDICATIONS 4
INDICATIONS 4
CONTRAINDICATIONS 4
ADDITIONAL PRECAUTIONS 4
ADVERSE EFFECTS 5
ULTRASOUND INDICATIONS 5
INDICATIONS 5
CONTRAINDICATIONS 5
ADDITIONAL PRECAUTIONS 5
GENERAL WARNINGS AND PRECAUTIONS 6
DEVICE DECSRIPTION 9
SYSTEM SOFTWARE SYMBOLS 9
DESCRIPTION OF DEVICE MARKINGS 10
PRODUCT DESCRIPTION 11
OPERATOR INTERFACE 12
POWERING UP THE DEVICE 14
DATA SYNCRHONISATION 15
SYSTEM 16
SYSTEM SPECIFICATIONS AND DIMENSIONS 16
WAVEFORMS 18
ELECTROTHERAPHY PATIENT PREPARATIONS AND
ELECTRODE PLACEMENT 22
ULTRASOUND PATIENT PREPARATION 23
DEVICE USER INTERFACE 24
SCREEN DESCRIPTION 24
SETTINGS 26
HOME SCREEN
27
TREATMENT REVIEW SCREEN 28
GUIDELINES SCREEN 29
ELECTROTHERAPY OPERATION 30
ULTRASOUND OPERATION 32
COMBINATION OPERATION 33
SPS (SUGGESTED PARAMETER SETUP) 35
TREATMENT DATA 36
CUSTOM PROTOCOLS 38
SHORT CUTS 39
CLINICAL RESOURCES 40
MODALITY/WAVEFORM DESCRIPTIONS 41
TROUBLESHOOTING 41
REPLACEMENT ACCESSORIES 42
CLEANING THE INTELECT® LEGEND 2 44
INSTRUCTION FOR SOFTWARE UPGRADE 44
WARRANTY 45
ELECTROMAGNETIC COMPATIBILITY
(EMC) TABLES 46
3INTRODUCTION EN
INTELECT®LEGEND 2 USER MANUAL
This manual is intended for users of the Intelect Legend
2 COMBO 2 CHANNEL (12-5010) and Intelect Legend
2 COMBO 4 CHANNEL (12-5011). It contains general
information on operation, precautionary practices, and
maintenance.
In order to maximize use, efficiency, and the life of the
system, please read this manual thoroughly and become
familiar with the controls, as well as the accessories before
operating the system.
Before administering any treatment to a patient, the
users of this equipment should read, understand, and
follow the information contained in this manual for each
mode of treatment available, as well as the indications,
contraindications, cautions, warnings, and dangers.
INTENDED PURPOSE
The Intelect® Legend 2 devices comprise of a range
of multimodality (TENS; NMES, Ultrasound) therapies
intended to be used by healthcare professionals using
TENS, NMES and Therapeutic Ultrasound for the treatment
of various musculoskeletal and skeletal muscle deficit
disorders.
The Intelect® Legend 2 product range offers the following
models:
The Intelect® Legend 2 2 Channel Combo device delivering
both 2 Channel Electrotherapy (TENS and NMES)
and Therapeutic Ultrasound either simultaneously or
independently.
The Intelect® Legend 2 4 Channel Combo device delivering
both 4 Channel Electrotherapy (TENS and NMES)
and Therapeutic Ultrasound either simultaneously or
independently.
INTENDED USER
The intended user of this device is a licensed healthcare
professional. The user should be able to:
• Read and understand the operator’s manual,
warnings, cautions and dangers.
• Sense auditory and visual signals.
• Read and understand indications and
contraindications of the device
FOREWORD
NOTE: Throughout this manual, “NOTE”
indicators provide helpful information
regarding the particular area of function
being described.
Text with a “CAUTION” indicator explains possible safety
infractions that have potential to cause minor or moderate
injury or damage to the equipment.
Text with a “WARNING” indicator explains possible safety
infractions that will potentially cause serious injury and
equipment damage.
Text with a “DANGER” indicator will explain possible safety
infractions that are imminently hazardous situations that
would result in death or serious injury.
The precautionary instructions found in this section and
throughout this manual are indicated by specific symbols.
Understand these symbols and their definitions before
operating this equipment. The definition of these symbols
are as follows:
PRECAUTIONARY INSTRUCTIONS
INTENDED ENVIRONMENT FOR USE
The device is intended to be operated in a professional
healthcare environment.
INTENDED PATIENT POPULATION
The Intelect® Legend 2 Devices are suitable for
adult patients requiring symptomatic treatment of
musculoskeletal conditions mentioned under “indications”
and to whom none of the contraindications apply.
WARNING
CAUTION
DANGER

4INDICATIONS FOR USE EN
INTELECT®LEGEND 2 USER MANUAL
INDICATIONS
For VMS (Pulsed Mode, Burst Mode, or FR Mode), Russian,
Monophasic Hi-Volt (NMES) & Interferential, and Premodulated
(IFS):
• Relaxation of muscle spasms
• Prevention or retardation of disuse atrophy
• Increasing local blood circulation
• Muscle re-education
• Maintaining or increasing range of motion
• Immediate postsurgical stimulation of calf muscles to
prevent venous thrombosis
Additionally for Microcurrent, Interferential, Premodulated (IFS),
VMS (Pulsed Mode, Burst Mode, or FR Mode), Asymmetrical
Biphasic (TENS), and HANS:
Symptomatic relief or management of chronic, intractable pain
• Post-traumatic acute pain
• Post-surgical acute pain
For DC (Direct Current) Continuous Mode:
• Relaxation of muscle spasms
CONTRAINDICATIONS
The Intelect® Legend 2 should NOT be used under the following
conditions:
• Do not use for symptomatic local pain relief unless
etiology is established or unless a pain syndrome has
been diagnosed
• Do not use when cancerous lesions are present in the
treatment area
• Do not apply stimulation over swollen,
infected, inflamed areas or skin eruptions (e g ,
phlebitis,thrombophlebitis, varicose veins, etc)
• Do not use when patient is suspected or known to
have infectious disease and/or disease where it is
advisable, for general medical purposes, to suppress
heat or fevers
• Do not place electrode placements to the carotid
sinus region (anterior neck) or transcerebrally
(through the head)
• Do not use on pregnant women, safety has not been
established for the use of therapeutic electrical
stimulation during pregnancy
• Do not use powered muscle stimulators or TENS
waveforms on patients with cardiac demand
pacemakers
• There should not be any use of TENS waveforms on
patients with cardiac demand pacemakers
• Do not use device on patients who have or have
had implantable neurostimulating cardiac demand
pacemakers, ICD, or other implantable electronic
devices
• Do not use device on patients with body worn electro
mechanical medical devices, i.e. insulin pump
• Do not use this system in an MRI or CT environment.
Device, its components, and accessories are not to
be present in an MRI or CT environment
ADDITIONAL PRECAUTIONS
• Use caution for patients with suspected or
diagnosed heart problems
• Use caution for patients with suspected or
diagnosed epilepsy
• Use caution in the presence of the following:
»When there is a tendency to
hemorrhage following acute trauma or
fracture
»Following recent surgical procedures
when muscle contraction may disrupt
the healing process
»Over a menstruating or pregnant uterus
»Over areas of the skin that lack normal
sensation
• Some patients may experience skin irritation or
hypersensitivity due to the electrical stimulation
or
electrical conductive medium. The irritation
can usually be reduced by using an alternative
conductive medium or an alternative electrode
placement
• Electrode placement and stimulation settings
should be based on the guidance of the
prescribing practitioner
• Powered muscle stimulators should be used only
with the lead wires and electrodes recommended
for use by the manufacturer
• With TENS waveforms, isolated cases of skin
irritation may occur at the site of electrode
placement following long-term application
• The effective management of pain by TENS
waveforms is highly dependent upon patient
selection by a person qualified in pain
management
ELECTROTHERAPY INDICATIONS

5INDICATIONS FOR USE EN
INTELECT®LEGEND 2 USER MANUAL
ELECTROTHERAPY INDICATIONS
(CONTINUED)
ADVERSE EFFECTS
• Skin irritation and burns beneath the electrodes
have been reported with the use of powered
muscle stimulators.
• Potential adverse effects with TENS are skin
irritation and electrode burns
INDICATIONS
Application of therapeutic deep heat for the treatment of
selected sub-chronic and chronic medical conditions such
as:
• Relief of pain, muscle spasms, and joint
contractures
• Relief of pain, muscle spasms, and joint
contractures that may be associated with:
»Adhesive capsulitis
»Bursitis with slight calcification
»Myositis
»Soft tissue injuries
»Shortened tendons due to past injuries
and scar tissues
• Relief of sub-chronic and chronic pain and joint
contractures resulting from:
»Capsular tightness
»Capsular scarring
CONTRAINDICATIONS
• Do not use for symptomatic local pain relief
unless etiology is established or unless a pain
syndrome has been diagnosed
• Do not use when cancerous lesions are present in
the treatment area
• Do not use when patient is suspected or known
to have infectious disease and/or disease where
it is advisable, for general medical purposes, to
suppress heat or fevers
• This device should not be used over or near bone
growth centers until bone growth is complete
• This device should not be used over the thoracic
area if the patient is using a cardiac pacemaker
• This device should not be used over a healing
fracture
• This device should not be used over or applied to
the eye
• This device should not be used over a pregnant
uterus
• Tissue necrosis might result if the device is used
on ischemic tissues in individuals with vascular
disease, where the blood supply would not keep
up with the metabolic demand
• Do not use device on patients who have or have
had implantable neurostimulating cardiac demand
pacemakers, ICD, or other implantable electronic
devices
• Do not use device on patients with body worn
electro mechanical medical devices, i.e. insulin
pump
• Do not use this system in an MRI or CT
environment. The device, its components, and
accessories are not to be present in an MRI or CT
environment
ADDITIONAL PRECAUTIONS
Additional precautions should be used when ultrasound is
used on patients with the following conditions:
• Over an area of the spinal cord following a
laminectomy, i.e. when major covering tissues
have been removed
• Over anesthetic areas
• On patients with hemorrhagic diatheses
ULTRASOUND INDICATIONS

6GENERAL WARNINGS AND PRECAUTIONS EN
INTELECT®LEGEND 2 USER MANUAL
CAUTION
• Do not operate this unit when connected to any unit other than DJO devices or accessories specifically described in
user or service manuals
• DO NOT disassemble, modify, or remodel the unit or accessories. This may cause unit damage, malfunction, electrical
shock, fire, or personal injury.
• Failure to use and maintain the device, its modules, and its accessories in accordance with the instructions outlined in
this manual will invalidate the warranty.
• DO NOT permit foreign materials, liquids, or cleaning agents (including, but not limited to, inflammables, water, and
metallic objects) to enter the unit to prevent unit damage, malfunction, electrical shock, fire, or personal injury.
• If you have difficulty operating the unit after carefully reviewing this user manual, contact your DJO dealer for
assistance
• DO NOT remove the cover. Doing so may cause unit damage, malfunction, electrical shock, fire, or personal injury.
There are no user-serviceable parts inside the unit. If a malfunction occurs, discontinue use immediately and consult
dealer for repair service.
• Use of parts or materials other than DJO’s can degrade minimum safety
• The device is not designed to prevent the ingress of water or liquids. Ingress of water or liquids could cause
malfunction of internal components of the system and therefore create a risk of injury to the patient
• DO NOT operate the device within the vicinity or environment as any microware and RF shortwave diathermy system
• DO NOT operate the device within the vicinity or environment as an ultrasonic diathermy system. The Ultrasound
(diathermy) Module of the device does not require separation distance
• DO NOT use electrodes with an active area less than 7.92 cm2, as there will be a risk of suffering a burn injury. Always
exercise caution with current densities more than 2mA/cm2.
• Ultrasound applied part is Type B. Its output is not isolated from the secondary voltage of the device. Make sure both
the mains connection and the applicator are properly connected to the device.
• Read, understand, and practice the precautionary and operating instructions. Know the limitations and hazards
associated with using any electrical stimulation, or ultrasound device. Observe the precautionary and operational
decals placed on the unit.
• All modalities should be routinely checked before each use to determine that all controls function normally, especially
that the intensity control does properly adjust the intensity of the ultrasonic power output in a stable manner. Also,
determine that the treatment time control does actually terminate ultrasonic power output when the timer reaches
zero.
• DO NOT use sharp objects such as a pencil point or ballpoint pen to operate the buttons on the control panel.
• This unit should be operated at 10°C to 40°C and 30% to 75% relative humidity The unit should be transported and
stored at -20°C to 60°C and 10°C to 90°C relative humidity
• Handle Ultrasound Applicator with care. Inappropriate handling may adversely affect its characteristics
• Before each use, inspect Ultrasound Applicator for cracks, which may allow the ingress of conductive fluid
• Inspect Applicator cables and associated connectors before each use
• Device is designed to comply with electromagnetic safety standards. This equipment generates, uses, and can radiate
radio frequency energy and, if not installed and used in accordance with instructions, may cause harmful interference
to other devices in the vicinity. However, there is no guarantee that interference will not occur in a particular
installation. Harmful interference to other devices can be determined by turning this equipment on and off. Try to
correct the interference using one or more of the following:
»Reorient or relocate the receiving device
»Increase the separation between the equipment
»Connect the equipment to an outlet on a different circuit from that to which the other device(s) are connected
»Consult your authorized DJO dealer for help

7GENERAL WARNINGS AND PRECAUTIONS EN
INTELECT®LEGEND 2 USER MANUAL
WARNING
• U S A Federal Law restricts these devices to sale by, or on the order of, a physician or licensed practitioner. This
device should be used only under the continued supervision of a physician or licensed practitioner.
• Be sure to read all instructions for operation before treating patient
• Make certain the unit is electrically grounded by connecting only to a grounded electrical service receptacle
conforming to the applicable national and local electrical codes
• Care must be taken when operating this equipment around other equipment. Potential electromagnetic or other
interference could occur to this or to the other equipment. Try to minimize this interference by not using other
equipment in conjunction with it.
• The safety of TENS waveforms for use during pregnancy or birth has not been established
• TENS is not effective for pain of central origin (This includes headache )
• TENS waveforms have no curative value
• Electronic monitoring equipment (such as ECG monitors and ECG alarms) may not operate properly when electrical
stimulation is in use.
• TENS is a symptomatic treatment, and as such, suppresses the sensation of pain which would otherwise serve as a
protective mechanism
• Do not drop the applicator or unit on hard surfaces or submerge in water. These actions will damage the applicator
and unit. Damage resulting from these conditions is not covered under the warranty.
• This device should be kept out of the reach of children
• Use of other accessories other than those specified in this User Manual may increase electrical emissions and
decrease electrical immunity of the device
• Contaminated sponges, electrodes, lead wires, and gel can lead to infection
• Use of electrode with degraded hydrogel can result in burn to the skin
• DO NOT operate this unit in an environment where other devices are being used that intentionally radiate
electromagnetic energy in an unshielded manner.
• Use of electrode on multiple patients can lead to infection
• Clean applicators after each use, otherwise it can lead to cross contamination and infection
• Do not treat through clothing
• Stop treatment immediately if patient experiences discomfort or pain
• Powered muscle stimulators should be used only with the leads and electrodes recommended for use by the
manufacturer
• Use of controls or adjustments or performance of procedures other than those specified herein may result in
hazardous exposure to ultrasonic energy
• Before administering any treatment to a patient you should become acquainted with the operating procedures for
each mode of treatment available, as well as the indications, contraindications, warnings, and precautions. Consult
other resources for additional information regarding the application of each mode of treatment
• Disconnect the system from the power source before attempting any maintenance, installation, removal, or
replacement procedures to prevent electrical shock and possible damage to system.
• Keep electrodes separated during treatment. Electrodes in contact with each other could result in improper
stimulation or skin burns.
• Long term effects of chronic electrical stimulation are unknown
• Stimulation should not be applied over the anterior neck or mouth Severe spasm of the laryngeal and pharyngeal
muscles may occur and the contractions may be strong enough to close the airway or cause difficulty in breathing
• Stimulation should not be applied transthoracically in that the introduction of electrical current into the heart may
cause cardiac arrhythmia
• Stimulation should not be applied over swollen, infected, and inflamed areas or skin eruptions, e g , phlebitis,
thrombophlebitis, varicose veins, etc
• Stimulation should not be applied over, or in proximity to, cancerous lesions
• Electrotherapy output current density is related to electrode size. Improper application may result in patient injury. If
any question arises as to the proper electrode size, consult a licensed practitioner prior to therapy session.
• The device's optional modules and associated accessories are designed for use only with this device
• Remove the Ultrasound by pulling the cable connector only DO NOT remove by pulling the cable
• Output current density is related to electrode size. Improper application may result in patient injury If any question
arises as to the proper electrode size, consult a licensed practitioner prior to therapy session.
• Medical electrical equipment needs special precautions regarding EMC Portable and mobile RF communication
equipment can be affected by other medical electrical devices. If you believe interference is occurring, please consult
the ELECTROMAGNETIC COMPATIBILITY (EMC) section to assist in removing the interference.
• Common RF emitting devices (e g , RFID) and electromagnetic security systems (e g , metal detectors) may interfere
with the operation of the device. The device has been tested in the presence of these types of devices and while no
adverse event occurred, the device should not be operated within the vicinity or environment as another RF emitting
device.
• The ultrasound applicator included in this set can expose you to chemicals including lead/lead components and
Bisphenol A (BPA) which are known to the state of California to cause cancer, birth defects or other reproductive
harm. For more information go to www.p65Warnings.ca.gov.
• The electro stimulation lead wires included in this set can expose you to chemicals including lead/lead components
which are known to the state of California to cause cancer, birth defects or other reproductive harm. For more
information go to www.p65Warnings.ca.gov.

8GENERAL WARNINGS AND PRECAUTIONS EN
INTELECT®LEGEND 2 USER MANUAL
DANGER
• Stimulus delivered by the TENS waveforms of this device, in certain configurations, will deliver a charge of 25
microcoulombs (µC) or greater per pulse and may be sufficient to cause electrocution. Electrical current of this
magnitude must not flow through the thorax because it may cause a cardiac arrhythmia.
• Patients with an implanted neurostimulation device must not be treated with or be in close proximity to any shortwave
diathermy, therapeutic ultrasound diathermy, anywhere on their body. Energy from diathermy (shortwave, microwave,
and ultrasound) can be transferred through the implanted neurostimulation system, can cause tissue damage, and
can result in severe injury or death Injury, damage, or death can occur during diathermy therapy even if the implanted
neurostimulation system is turned off.
• Handle, clean, and dispose of components and accessories that have come in contact with bodily fluids according to
National, Local, and Facility rules, regulations, and procedures.
• The solvents of adhesives and flammable solutions used for cleaning and disinfecting should be allowed to evaporate
before the unit is used.
• DO NOT connect the unit to an electrical supply without first verifying that the power supply is the correct voltage.
Incorrect voltage may cause unit damage, malfunction,electrical shock, fire, or personal injury. Your unit was
constructed to operate only on the electrical voltage specified on the Voltage Rating and Serial Number Plate. Contact
your DJO dealer if the unit is not properly rated
• Device is not designed to be used in oxygen rich environment. Explosion hazard if the device is used in the presence of
flammable anesthetic mixture with air, oxygen, or nitrous oxide.
• Charge the Battery Module according to the instructions found in this manual. Never attempt to charge the Battery
Module on any other charging mechanism.
• Do not reverse the polarity of the Battery Module. Doing so can increase the individual cell temperature and cause
cell rupture or leakage.
• Never dispose of Battery Module in fire. Never short circuit the battery. The battery may explode, ignite, leak or get hot
causing serious personal injury.
• Dispose of batteries according to national, state and local codes and regulations.
9GENERAL WARNINGS AND PRECAUTIONS EN
INTELECT®LEGEND 2 USER MANUAL
GENERAL TERMINOLOGY
The following are definitions for the terminology used throughout this manual . Study these terms to become familiar with them
for ease of system operation and control functionality of the Intelect® Legend 2 .
SYSTEM SOFTWARE SYMBOLS
Home
Back to previous screen
Settings
Indicates a USB Flash Drive is Inserted
Indicates Battery Level
Indicates more content can be
viewed by swiping vertically
Indicates more content can be
viewed by swiping horizontally
Indicates more content can be
viewed by scrolling
Close window / exit full screen
Confirm
Save Data
Edit
Guidelines / Assign to
Pain information
Setup
Running
Paused
Completed
Run again
Exit
Export
Import
Delete
Delete all
Stop treatment
Stim
Ultrasound
Combo
Shortcut
SPS (Suggested
Parameter Setup)
Custom Protocols
Treatment Data
Clinical Resources

10 GENERAL WARNINGS AND PRECAUTIONS EN
INTELECT®LEGEND 2 USER MANUAL
DESCRIPTION OF DEVICE MARKINGS
The markings on the unit are assurance of its conformity to
the latest applicable standards of medical equipment safety
and electromagnetic compatibility and conform to ISO 7010
and ISO 15223-1 One or more of the following markings may
appear on the device:
Storage conditions
Temperature Range
Relative Humidity Range
Atmospheric Pressure Range
Test agency
CE Mark of Conformity with
notified body number
Alternating current
IP20
IP20
Radio frequency equipment
WEEE Directive conformity
Shelf life
Batch number
US amplitude modulated
MD
Unique Device Identification
Federal Law restricts this unit
to sale by, or on the order of, a
physician or licensed practitioner.
Note: This equipment is to be
used only by a licensed medical
practitioner.
Consult Instruction for Use
Follow Instructions for Use
Warning, Caution, or Danger
Electrical Type BF Equipment
Electrical Type B Equipment
Ultrasound
Rechargeable
Stim
Combo
Play
Pause
ON/OFF
Manufacturer
Date of manufacture
Catalogue number
Serial number
Fragile, handle with care
This end up
Keep dry
SN
REF

11 DEVICE DESCRIPTION EN
INTELECT®LEGEND 2 USER MANUAL
PRODUCT DESCRIPTION
The Intelect® Legend 2 is a two-channel or 4-channel
electrotherapy and ultrasound combo system used with
or without an optional Cart, allowing for the inclusion of a
Vacuum module. This equipment is to be used only under
the prescription and supervision of a licensed healthcare
professional.
HEAD
2 CHANNEL COMBO SET INCLUDES:
12-5000 Intelect Legend 2 Two Channel Combo
70010 STIM lead wires CH 1 & 2
12-10648 Nylatex strap
14679 Power cord
42198 Dura-Stick+ 5cm square electrodes,
QTY 4
79967 Carbon electrodes
15-0162 5cm2 Ultrasound Applicator
15-1140 USB Drive
4 CHANNEL COMBO SET INCLUDES:
12-5001
Intelect Legend 2 Four Channel Combo
70010 STIM lead wires CH 1 & 2
70011 STIM lead wires CH 3 & 4
12-10648 Nylatex strap
14679 Power cord
42198
Dura-Stick+ 5cm square electrodes, QTY 4
79967 Carbon electrodes
15-0162 5cm2 Ultrasound Applicator
15-1140 USB Drive
CART (OPTIONAL)
ULTRASOUND APPLICATORS
1. Applicator Head
The component of the applicator that makes contact with
the patient during Ultrasound or Combination therapy.
2. Applicator
The assembly that connects to the system and
incorporates the Applicator head.
3. LED
The component of the applicator that indicates if the
Applicator is coupled or uncoupled on the treatment area.
BATTERY MODULE (optional)
Battery (type ABI-L 18650-5S1P) is an 18.15V 3250mA
(58.98 Wh) Li-Ion rechargeable battery.
Operating temperature: 0°C to 45°C
Storage temperature (1month): -20°C to 60°C
Storage temperature (6months): -20°C to 45°C
Storage temperature (12months): -20°C to 25°C
12-5010
Intelect® Legend 2Two Channel Combo Device
12-5011
Intelect® Legend 2Four Channel Combo Device

12 DEVICE DESCRIPTION EN
INTELECT®LEGEND 2 USER MANUAL
OPERATOR INTERFACE
5. ON/OFF switch (only active when connected to the mains)
6. Ultrasound Applicator holder, left and right sides
7. Mains power connector
8. Battery cover
9. USB Flash Drive Port located inside battery trap
10. Magnetic fixation to the cart
11. Vacuum cover
12. Device handle
The Intelect® Legend 2Operator Interface contains all the functions
and controls necessary for operator access to all operator utilities,
modalities, and parameters for modification and system set up.
1. Color Display and touch screen
2. Adjustment dial
3. Play/pause button
4. “On/Off“ button. Press and hold (2sec) the button to switch OFF the
device.
Handle
Colour Touch
Screen
Play / pause
button
Adjustment dial
Ultrasound applicator
support
“On/Off“ button
Ultrasound applicator
connectors Lead wire connectors for
electro stimulation ON / OFF switch
only active on
mains power Mains power
connector
Ultrasound applicator
Battery cover Vacuum
cover

13 SETUP INSTRUCTIONS EN
INTELECT®LEGEND 2 USER MANUAL
DEVICE LIGHT INDICATORS
Intelect® Legend 2 has several light indicators:
FRONT PANEL INDICATORS:
1. Colors:
• Light blue around Ultrasound therapy channel Left
and Right
• Dark blue indicator around Electrostimulation
Channel 1
• Green indicator around Electrostimulation Channel 2
• Orange indicator around Electrostimulation Channel 3
• Red indicator around Electrostimulation Channel 4
2. Behavior:
• Steady when modality is selected and output is not
active
• Flashing when output is active
• Quickly flashing when treatment is interrupted and
user action is requested
ON/OFF BUTTON BLUE INDICATOR:
• Steady ON from device connection to the mains
• Flashing while powering ON/OFF
PLAY/PAUSE BUTTON BLUE INDICATOR:
• Flashes when user can start/resume a treatment.
Otherwise, steady.
HEAD TO CART FIXATION
The optional Therapy System Cart allows the user to easily
transport the System from patient to patient within the clinic
as well as store all necessary accessories, supplies, and
applicators used for the various modalities of the System.
The fixation of the head to the cart is magnetic.
Remove the Intelect® Legend 2 device and cart from the
shipping carton. Visually inspect for damage. Report any
damage to the carrier immediately. To assemble the Legend
2 Head to the Cart, follow these steps:
1. Insert device front bottom on the cart lip
2. Release device back gently on the cart. Magnets will help
to position the device correctly on the cart top.
IF UNIT SUPPLIED WITH OPTIONAL BATTERY
After unpacking Intelect® Legend 2 to fit the battery follow
the following steps
1. Unscrew the battery cover from the base of the device by
removing the 2 screws see below
2. Remove the battery cover
3. Plug the battery into the battery connector on the device
4. Insert the battery into its location
5. Replace the 2 screws to close the battery cover
Note: in case of unused device with the battery installed, it is
recommended to connect the device to the mains power
and power on the device with the main ON/OFF switch on
the back of the device at least once every 4 months to allow
the battery to recharge.
14 SETUP INSTRUCTIONS EN
INTELECT®LEGEND 2 USER MANUAL
DEVICE WORKING ON BATTERY
1. Press the ON/OFF button on the LCD Front panel.
2. Select desired function on the Home Screen.
STOP TREATMENT AND TURN OFF THE
DEVICE
Press Play/pause button to pause treatment then press stop
on touch screen. If device is on mains power press the on/
off button on the front panel then turn off the switch on the
back of the unit.
If device is working on battery follow the above procedure
but to switch off only press the on/off button on the front
panel
CONNECTING CABLES AND INSERTING
PLUGS
When inserting the plugs, be sure to align the flat side of the
plug with the flat side of the slot and push in gently. This is
to avoid bending the pins in the plug.
Insert cable into the appropriate connector prior to starting
therapy.
POWERING UP THE DEVICE
When powering up the device for the first time, always use
mains power even if a battery is connected. Insert the power
cord into the back of the unit, insert the plug into a power
outlet, do not position the Intelect® Legend 2 in such a way
that makes it difficult to disconnect from the mains power.
Switch device on with ON/OFF switch on the back of the unit
1. The Initialization screen below will be shown for a few
seconds whilst the device starts.
2. The first setup screen will be displayed after this allowing
the user to set language, device name, time and choose
patient pain scale as either NRS (Numerical Rating Scale) or
VAS (Visual Analogue Scale).
3. Click on "Continue" button to go to home screen
DEVICE CONNECTED TO THE MAINS
1. Plug the Power cord into the back of device. Plug the other
end of the cord into an electrical outlet.
NOTE: The Power Cord may be unplugged from the back of
the unit in an emergency situation.
2. Turn on the ON/OFF switch located on the back of the
device.
3. Press ON/OFF button on LCD Front panel
4. Select desired function on the Home Screen
Intelect Legend 2

15 SETUP INSTRUCTIONS EN
INTELECT®LEGEND 2 USER MANUAL
DATA SYNCHRONIZATION
Chattannoga Intelect® Connect App is an optional software that can be installed on a computer. It uses Bluetooth® low energy to
connect to the device to provide the following features:
1. Import/Export Custom Protocols
2. Import/Export Treatment data
3. Import Sessions of the device on the computer
4. Archive the Treatment Data's session history in a format that can be used for reporting
5. Backup/Restore device configuration
Refer to Chattanooga website to download it, Microsoft Windows 10 or higher with Bluetooth® Low Energy communication
capabilities computer is required.
Note: device can not be used to deliver treatment while data transfer.
To prepare for communication with the App press the settings button, scroll down the screen and press the Data transfer
button.
You should now see a screen that says "Waiting for connection..." whilst the device discovers the computer in which it can
connect.
Start the Chattanooga Intelect® connect App and follow instructions on computer screen.

16 SYSTEM EN
INTELECT®LEGEND 2 USER MANUAL
POWER
Input 100 - 240 V AC, 1.0 to 0.42 A, 50/60 Hz
Electrical Class CLASS I
Mode of Operation Continuous
Note: Mains isolation is achieved by use of the double
pole switch located on the rear panel.
Electrical Type (Degree of Protection)
Ultrasound .TYPE B
Electrotherapy .TYPE BF
ELECTRO STIMULATION SPECIFICATIONS
Output specifications are described for each waveform
from pages 18-21.
Unless otherwise specified, electrotherapy controls
accuracy is: ± 20 %.
Load impedance: 500-1000 Ohm
CC = constant current, effect of load impedance on
voltage
CV = constant voltage, effect of load impedance on
current
ULTRASOUND SPECIFICATIONS
Frequency 1 MHz +/- 10%; 3 MHz +/- 10%
Duty Cycles 10%, 20%, 50%, Continuous
Pulse Repetition Rate 100 Hz
Pulse duration: 1 -5 ms
Max (ON): 5 ms
Min (OFF): 5ms
SYSTEM SPECIFICATIONS AND DIMENSIONS
Width Depth Height Weight (no battery)
Intelect® Legend 2 Head Unit
2 Channel COMBO 34cm 35.5cm 15cm 3.1kg
4 Channel COMBO 34cm 35.5cm 19cm 3.7kg
Cart configurations
Cart (Safe working load 6.5kg) 48cm (MAX) 52cm (MAX) 96cm 10.1kg
Device on cart - - 111 cm -
OUTPUT POWER
US applicator
Frequency
1cm22cm25cm210cm2
1MHz 3MHz 1MHz 3MHz 1MHz 3MHz 1MHz 3MHz
Effective
Radiating
Area ERA
INTL (cm2)
1 0.9 1.5 1 2.5 2.7 6 6.8
Max Output
power in
Continuous
mode
2W 1.8W 3W 2W 5W 5.4W 12W6.8W
Max Output
power in
Pulsed
mode
3W 2.7W (*) 4.5W 3W 7.5W 8.1W 18W13.6W
Max
Amplitude in
Continuous
mode
2W/
cm2
2W/
cm2
2W/
cm2
2W/
cm2
2W/
cm2
2W/
cm2
2W/
cm2
1W/
cm2
Max
Amplitude in
Pulsed mode
3W/
cm2
3W/
cm2
3W/
cm2
3W/
cm2
3W/
cm2
3W/
cm2
3W/
cm2
2W/
cm2
(*) An error of + 0.25 W can be measured with 1cm2US applicator, pulse mode
100Hz at 10% or 20% Duty Cycle.
Unless otherwise specified, ultrasound
controls accuracy is: ± 20 %.
Peak to Average Ratio: 1:1, at 50% Duty Cycle
4:1, at 20% Duty Cycle
9:1, at 10% Duty Cycle
Beam Nonuniformity Ratio <5:1
Beam Type Collimating
Treatment Time 1to 30 min

17 SYSTEM EN
INTELECT®LEGEND 2 USER MANUAL
GENERAL SYSTEM OPERATING AND STORAGE
TEMPERATURE
Operating Conditions
The device will meet its requirement under the following
conditions:
Temperature: 10°C to 40°C
Relative Humidity: 30% to 75%
Atmospheric Pressure: 70kPa to 106kPa
Transport and Storage Conditions
The device will remain in proper condition under the following
conditions:
Temperature: -20°C to 60°C
Relative Humidity: 10% to 90%
Atmospheric Pressure: 50kPa to 106kPa
Time required for the Intelect® Legend 2 to warm from the
minimum storage temperature between uses until the Intelect®
Legend 2 is ready for its INTENDED USE when the ambient
temperature is 20 °C: 5h
Time required for the Intelect® Legend 2 to cool from the
maximum storage temperature between uses until the
Intelect® Legend 2 is ready for its INTENDED USE when the
ambient temperature is 20 °C: 5h
IPXX Rating for Unit
Rated to IP20
IP2* Protection against fingers or other object not greater than
80mm in length and 12mm in diameter
*0 No Special Protection Against Liquids
IPXX Rating for US applicator
Rated to IPX7
IPX7 Protection from immersed in water (up to 1m depth)
RED
RF transmitter/receiver characteristics:
- Frequency Band transmission: 2400–2483.5 MHz
- Modulation type: GFSK
- Data rate: up to 2Mbps 500kHz deviation at 2Mbps
- Effective radiated power: +6dBm
18 SYSTEM EN
INTELECT®LEGEND 2 USER MANUAL
Advice on size and type of electrodes to be used is given
in device User Interface treatment guidelines.
CC: Constant Current CV: Constant Voltage
WAVEFORMS
IFC (Interferential) Traditional (4Pole)
Advice on size and type of electrodes to be used is given in
device GUI "treatment guidelines" feature
Interferential Current is a medium frequency waveform.
Current is distributed through two channels (four
electrodes). The currents cross each other in the body at
the area requiring treatment. The two currents interfere
with each other at this crossing point, resulting in a
modulation of the intensity (the current intensity increases
and decreases at a regular frequency).
Output Mode Electrodes
Available on Channel 1 & 2, 3 & 4
Treatment Time 1-60 Minutes
Mode Selection CC
Output Intensity 0-100 mA (CC)
Beat Frequency 1-200 Hz
Carrier Frequency 2000-10,000 Hz
Cycle Time Continuous or User Defined
Sweep Time 14 sec
Sweep Low Beat Frequency 1-199 Hz
Sweep High Beat Frequency 2-200 Hz
Scan Percentage Static, 40%, 100%, Manual
IRMS 0-78mA
DC component No
TENS- Asymmetrical Biphasic
Advice on size and type of electrodes to be used is given in
device GUI "treatment guidelines" feature
The Asymmetrical Biphasic waveform has a short pulse
duration. It is capable of strong stimulation of the nerve
fibers in the skin as well as of muscle tissue. This waveform
is often used in TENS devices.Because of its short pulse,
the patient typically tolerates the current well, even at
relatively high intensities.
Output Mode Electrodes
Output Intensity 0-140 mA (CC) 0-140 V (CV)
Available on Channel 1, 2, 3, 4
Treatment Time (Stim) 1-60 minutes
Treatment Time (Combo) 1-30 minutes
Mode Selection (Stim) CC or CV
Mode Selection (Combo) CV
Amplitude Modulation 0% (off) to 100% on 10% steps
Burst Frequency 0-10 bps
Cycle Time Continuous or User Defined
Frequency 1-200 pps
FrequencySweep On/Off
Phase Duration 30-400 μsec
Sweep time 14 sec
Sweep Low Frequency 1-199 pps
Sweep High Frequency 2-200 pps
IRMS 0-50mA
DC component No
TENS- Symmetrical Biphasic
Advice on size and type of electrodes to be used is given in
device GUI "treatment guidelines" feature
The Symmetrical Biphasic waveform has a short pulse
duration and is capable of strong stimulation of nerve fibers
in the skin and in muscle. This waveform is often used in
portable muscle stimulation units, and some TENS devices.
Output Mode Electrodes
Available on Channel 1, 2, 3, 4
Treatment Time (Stim) 1-60 min
Treatment Time (Combo) 1-30 minutes
Mode Selection (Stim) CC or CV
Mode Selection (Combo) CV
Output Intensity 0-140 mA (CC) 0-140 V (CV)
Amplitude Modulation 0% (off) to 100% on 10% steps
Burst Frequency 0-10 bps
Cycle Time Continuous or User Defined
Frequency 1-200 pps
Frequency Sweep On/Off
Phase Duration 30-400 μsec
Ramp 0-5 sec
Sweep Time 14sec
Sweep Low Frequency 1-199 pps
Sweep High Frequency 2-200 pps
IRMS 0-50mA
DC component No

19 SYSTEM EN
INTELECT®LEGEND 2 USER MANUAL
WAVEFORMS (CONTINUED)
TENS - HAN
Advice on size and type of electrodes to be used is given in
device GUI "treatment guidelines" feature
The HAN Waveform provides optimal parameters with a
precisely controlled sequence of Dense-and-Disperse
(DD) modes of stimulation where a burst of 8 pulses
at 80Hz is alternating with continuous stimulation (no
burst), each lasting for 3 seconds.
Output Mode Electrodes
Available on Channels 1, 2, 3, 4
Treatment Time 1-60 min
Mode Selection CC
Output Intensity 0-100 mA (CC)
Burst Frequency 2 bps
Frequency 80 pps
Phase Duration 180 μsec
IRMS 0-19mA
DC component No
Microcurrent
Advice on size and type of electrodes to be used is given in
device GUI "treatment guidelines" feature
Microcurrent is a monophasic waveform of very low
intensity.
Output Mode Electrodes
Available on channels 1, 2, 3, 4
Treatment Time 1-60 Min
Mode Selection CC
Output Intensity 0-1,000 μA
Duty Cycle 50%
Frequency 0.1-1,000 pps
Polarity Positive, Negative, or Alternating
IRMS 0- 1mA
DC component No
VMS™
Advice on size and type of electrodes to be used is given in
device GUI "treatment guidelines" feature
VMS is a symmetrical biphasic waveform with a 100 sec
interphase interval. Because the pulse is relatively short,
the waveform has a low skin load, making it suitable for
applications requiring high intensities, such as in muscle
re-education protocols.
Output Mode Electrodes
Available on Channels 1, 2, 3, 4
Treatment Time (Stim) 1-60 min
Treatment time (Combo) 1-30 min
Mode Selection CC or CV
Output Intensity 0- 140 mA (CC) 0-140 V (CV)
Anti-Fatigue Off or On
Channel Mode Single, Reciprocal, Co-Contract
Cycle Time Continuous or User Defined
Frequency 1-200 pps
Phase Duration 30-1,000 μsec
Ramp 0-5 sec
Set Intensity Individual/both Channel Intensity
Setting in Reciprocal and Co-Contract modes
IRMS 0-50mA
DC component No
20 SYSTEM EN
INTELECT®LEGEND 2 USER MANUAL
WAVEFORMS (CONTINUED)
IFC Premodulated (Traditional 2 Pole)
Advice on size and type of electrodes to be used is given in
device GUI "treatment guidelines" feature
Premodulated Current is a medium frequency waveform.
Current comes out of one channel (two electrodes). The
current intensity is modulated: it increases and decreases
at a regular frequency (the Amplitude Modulation
Frequency).
Output Mode Electrodes
Available on Channel 1, 2, 3, 4
Treatment Time (STIM) 1-60 Min
Treatment Time (COMBO) 1-30 Min
Mode Selection CC or CV
Output Intensity 0-100 mA (CC) 0-100 V (CV))
Carrier
Beat Fixed (Sweep Off) 1-200 Hz
Cycle Time Continuous or User Defined
Frequency 2,000-10,000 Hz
Sweep Low Beat Frequency 1- 199 Hz
Sweep High Beat Frequency 2-200 Hz
IRMS 0-55mA
DC component No
Russian
Advice on size and type of electrodes to be used is given in
device GUI "treatment guidelines" feature
Russian Current is a sinusoidal waveform, delivered in
bursts or series of pulses.
Output Mode Electrodes
Available on Channels 1, 2, 3, 4
Treatment Time 1-60 min
Mode Selection CC or CV
Output Intensity 0-100 mA (CC) 0-100 V (CV)
Burst Frequency 1-100 bps
Carrier Frequency 2,500 Hz
Cycle Time Continuous or User Defined
Duty Cycle 10%, 20%, 30%, 40%, 50%
Ramp 0-5 sec
IRMS 0-39mA
DC component
V
MS™ Burst
Advice on size and type of electrodes to be used is given in
device GUI "treatment guidelines" feature
VMS Burst is a symmetrical biphasic waveform delivered
in a burst format. Because the pulse is relatively short,
the waveform has a low skin load, making it suitable for
applications requiring high intensities, such as muscle re-
education protocols.
Output Mode Electrodes
Available on Channels 1, 2, 3, 4
Treatment Time 1-60 min
Mode Selection CC or CV
Output Intensity 0-140 mA (CC) 0-140 V (CV)
Anti- Off or On
Burst Frequency 1-200 bps
Channel Mode Single, Reciprocal, Co-Contract
Phase
Cycle Time Continuous or User Defined
Duration 30-400 μsec
Ramp 0-5 sec
Set Intensity Individual/both Channel Intensity
Setting in Reciprocal and Co-Contract modes
IRMS 0-50mA
DC component No
DC (Direct Current)
Advice on size and type of electrodes to be used is given in
device GUI "treatment guidelines" feature
Galvanic Current is a direct current flowing in one
direction only.
The current can be continuous or interrupted.
Output Mode Electrodes
Available on Channels 1, 2, 3, 4
Treatment Time 1-60 min
Mode Selection CC
Output Intensity 0-40 mA (CC)
Cycle Time Continuous, or User Defined
Polarity Reversal On or Off
With Polarity Reversal On, Polarity will change in
the middle of the treatment time.
IRMS 0-44mA
DC component Yes
This manual suits for next models
2
Table of contents
Languages:
Other Intelect Medical Equipment manuals
Popular Medical Equipment manuals by other brands

EIC
EIC transfusio-therm 2000 Quick reference guide

Inogen
Inogen One G4 Getting started guide

Lowenstein Medical
Lowenstein Medical prismaPSG Instructions for use

Omron
Omron M2 Compact New Product Information Sheet
Stryker
Stryker SYSTEM G 7305-001-000 Instructions for use
Mindray
Mindray V Series Quick reference guide