Intelect TENS User manual

Intelect IFC
®
Intelect TENS
®
Intelect TENS
®
Intelect TENS
®
2003 Encore Medical
2003 Encore Medical
2003 Encore Medical
2003 Encore Medical
2003 Encore Medical
NMES
®
Intelect
NMES
®
Intelect
77393A
77395A
77684C
77392A
77391A
77394A

Chapter Page
4.7 Adjust the Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
4.8 Adjust Timer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
4.9 Adjust Channel Amplitude . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
4.10 Turn Unit Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
4.11 Portability . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
4.12 Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
4.13 Care of Electrodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
4.14 Care of Electrode cords . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
5 HANDLING AND STORAGE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
6 SPECIFICATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
7 ACCESSORIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .16
8 TROUBLESHOOTING . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .17
9 WARRANTY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .18
Chapter Page
1 INTRODUCTION
1.1 General information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
1.2 Caution . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
1.3 Indications for use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
1.4 Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-2
1.5 Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-3
1.6 Adverse Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
2 PRODUCT DESCRIPTIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-6
3 STIMULATION MODES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-8
4 INSTRUCTIONS FOR USE
4.1 Check Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
4.2 Connect electrodes to lead wires . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
4.3 Connect lead wires to unit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
4.4 Place electrodes on skin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
4.5 Select the mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
4.6 Adjust the Pulse Width . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
Contents

Chapter Page
4.7 Adjust the Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
4.8 Adjust Timer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
4.9 Adjust Channel Amplitude . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
4.10 Turn Unit Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
4.11 Portability . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
4.12 Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
4.13 Care of Electrodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
4.14 Care of Electrode cords . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
5 HANDLING AND STORAGE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
6 SPECIFICATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
7 ACCESSORIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .16
8 TROUBLESHOOTING . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .17
9 WARRANTY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .18
Chapter Page
1 INTRODUCTION
1.1 General information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
1.2 Caution . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
1.3 Indications for use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
1.4 Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-2
1.5 Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-3
1.6 Adverse Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
2 PRODUCT DESCRIPTIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-6
3 STIMULATION MODES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-8
4 INSTRUCTIONS FOR USE
4.1 Check Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
4.2 Connect electrodes to lead wires . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
4.3 Connect lead wires to unit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
4.4 Place electrodes on skin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
4.5 Select the mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
4.6 Adjust the Pulse Width . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
Contents

12
1.4.5 Stimulation should not be applied over swollen, infected, or inflamed areas or skin eruptions, e.g., phlebitis,
thrombophlebitis, varicose veins, etc.
1.4.6 Stimulation should not be applied over, or in proximity to, cancerous lesions.
1.4.7 For external use only.
1.4.8 Do not use TENS on the eye area.
1.4.9 This device should be used only under the continued supervision of a licensed medical practitioner.
1.4.10 Safety of TENS devices for use during pregnancy or delivery has not been established.
1.4.11 Electronic equipment such as ECG monitors and ECG alarms may not operate properly when TENS is in use.
1.4.12 Apply the electrodes to clean, dry, and unbroken skin only.
1.4.13 This device should not be used while driving, operating machinery, or during any activity in which
involuntary muscle contractions may put the user at undue risk of injury.
1.4.14 This device should be kept out of the reach of children.
1.4.15 Keep electrodes separate during treatment. Electrodes in contact with each other could result in improper
stimulation or skin burns.
1.5 Precautions:
1.5.1 Caution should be used for patients with suspected or diagnosed heart problems.
1.5.2 Caution should be used for patients with suspected or diagnosed epilepsy.
1.5.3 Caution should be used in the presence of the following:
(a)When there is a tendency to hemorrhage following acute trauma or fracture
(b)Following recent surgical procedures when muscle contraction may disrupt the healing process.
1.1 General information:
This TENS is a lightweight and portable medical device which can help to reduce pain and discomfort. It utilizes low
electric-current to stimulate muscle nerves to achieve the symptomatic relief of chronic intractable pain, post-
traumatic and post-surgical pain.
1.2. Cautions
Federal law (USA) restricts this device to sale by or on the order of practitioners licensed by the State in which they
practice to use or order the use of the device.
1.3. Indications for use:
This device is used in symptomatic relief of chronic intractable pain, post-traumatic and post-surgical pain.
1.4 Warnings:
1.4.1 The long-term effects of chronic electrical stimulation are unknown.
1.4.2 Stimulation should not be applied over the carotid sinus nerves, particularly in patients with a known
sensitivity to the carotid sinus reflex.
1.4.3 Stimulation should not be applied over the neck or mouth. Severe spasm of the laryngeal and pharyngeal
muscles may occur and the contractions may be strong enough to close the airway or cause difficulty in
breathing.
1.4.4 Stimulation should not be applied transthoracically in that the introduction of electrical current into the
heart may cause cardiac arrhythmias.
1. INTRODUCTION

12
1.4.5 Stimulation should not be applied over swollen, infected, or inflamed areas or skin eruptions, e.g., phlebitis,
thrombophlebitis, varicose veins, etc.
1.4.6 Stimulation should not be applied over, or in proximity to, cancerous lesions.
1.4.7 For external use only.
1.4.8 Do not use TENS on the eye area.
1.4.9 This device should be used only under the continued supervision of a licensed medical practitioner.
1.4.10 Safety of TENS devices for use during pregnancy or delivery has not been established.
1.4.11 Electronic equipment such as ECG monitors and ECG alarms may not operate properly when TENS is in use.
1.4.12 Apply the electrodes to clean, dry, and unbroken skin only.
1.4.13 This device should not be used while driving, operating machinery, or during any activity in which
involuntary muscle contractions may put the user at undue risk of injury.
1.4.14 This device should be kept out of the reach of children.
1.4.15 Keep electrodes separate during treatment. Electrodes in contact with each other could result in improper
stimulation or skin burns.
1.5 Precautions:
1.5.1 Caution should be used for patients with suspected or diagnosed heart problems.
1.5.2 Caution should be used for patients with suspected or diagnosed epilepsy.
1.5.3 Caution should be used in the presence of the following:
(a)When there is a tendency to hemorrhage following acute trauma or fracture
(b)Following recent surgical procedures when muscle contraction may disrupt the healing process.
1.1 General information:
This TENS is a lightweight and portable medical device which can help to reduce pain and discomfort. It utilizes low
electric-current to stimulate muscle nerves to achieve the symptomatic relief of chronic intractable pain, post-
traumatic and post-surgical pain.
1.2. Cautions
Federal law (USA) restricts this device to sale by or on the order of practitioners licensed by the State in which they
practice to use or order the use of the device.
1.3. Indications for use:
This device is used in symptomatic relief of chronic intractable pain, post-traumatic and post-surgical pain.
1.4 Warnings:
1.4.1 The long-term effects of chronic electrical stimulation are unknown.
1.4.2 Stimulation should not be applied over the carotid sinus nerves, particularly in patients with a known
sensitivity to the carotid sinus reflex.
1.4.3 Stimulation should not be applied over the neck or mouth. Severe spasm of the laryngeal and pharyngeal
muscles may occur and the contractions may be strong enough to close the airway or cause difficulty in
breathing.
1.4.4 Stimulation should not be applied transthoracically in that the introduction of electrical current into the
heart may cause cardiac arrhythmias.
1. INTRODUCTION

3
2.1 Front and Rear panel:(c)Over the menstruating or pregnant uterus
(d)Over areas of the skin which lack normal sensation.
1.5.4 Some patients may experience skin irritation or hypersensitivity due to the electrical stimulation or electrical
conductive medium. The irritation can usually be reduced by using an alternate conductive medium, or
alternate electrode placement.
1.5.5 Electrode placement and stimulation settings should be based on the guidance of the prescribing
practitioner.
1.5.6 This device should be used only with the leads and electrodes recommended for use by the manufacturer.
1.5.7 Isolated cases of skin irritation may occur at the site of the electrode placement following long-term
application.
1.5.8 Effectiveness is highly dependent upon patient selection by a person qualified in the management of pain-
afflicted patients.
1.5.9 If the stimulation levels are uncomfortable or become uncomfortable, reduce the stimulation amplitude to a
comfortable level and contact your physician if problems persist.
1.6 Adverse Reactions:
1.6.1 Possible skin irritation or electrode burn under the electrodes may occur.
1.6.2 Possible allergic skin reaction to tape or gel may occur.
2. PRODUCT DESCRIPTIONS
4
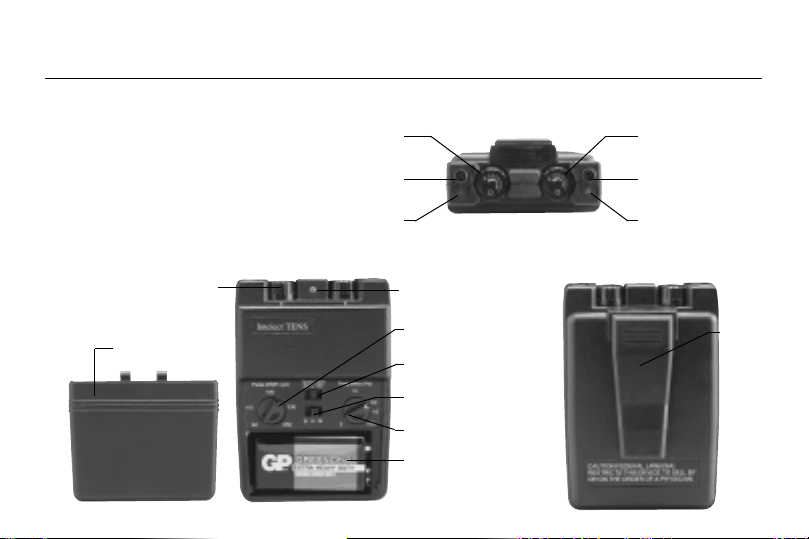
Front View
Back View
Top View Channel 1 On/Off and
Amplitude Control
Channel 1 Output
Receptacle
Channel 1 Output
Indicator Light
Channel 2 On/Off and
Amplitude Control
Channel 2 Output
Receptacle
Channel 2 Output
Indicator Light
Power Indicator
Light
Clip
Timer Switch
Frequency Control
Battery Compartment
Amplitude Control
Mode Switch
Pulse Width
Control
Lid Cover
3
2.1 Front and Rear panel:(c)Over the menstruating or pregnant uterus
(d)Over areas of the skin which lack normal sensation.
1.5.4 Some patients may experience skin irritation or hypersensitivity due to the electrical stimulation or electrical
conductive medium. The irritation can usually be reduced by using an alternate conductive medium, or
alternate electrode placement.
1.5.5 Electrode placement and stimulation settings should be based on the guidance of the prescribing
practitioner.
1.5.6 This device should be used only with the leads and electrodes recommended for use by the manufacturer.
1.5.7 Isolated cases of skin irritation may occur at the site of the electrode placement following long-term
application.
1.5.8 Effectiveness is highly dependent upon patient selection by a person qualified in the management of pain-
afflicted patients.
1.5.9 If the stimulation levels are uncomfortable or become uncomfortable, reduce the stimulation amplitude to a
comfortable level and contact your physician if problems persist.
1.6 Adverse Reactions:
1.6.1 Possible skin irritation or electrode burn under the electrodes may occur.
1.6.2 Possible allergic skin reaction to tape or gel may occur.
2. PRODUCT DESCRIPTIONS
4
Front View
Back View
Top View Channel 1 On/Off and
Amplitude Control
Channel 1 Output
Receptacle
Channel 1 Output
Indicator Light
Channel 2 On/Off and
Amplitude Control
Channel 2 Output
Receptacle
Channel 2 Output
Indicator Light
Power Indicator
Light
Clip
Timer Switch
Frequency Control
Battery Compartment
Amplitude Control
Mode Switch
Pulse Width
Control
Lid Cover

56
Mode Switch
Set Normal (N), Burst(B), or Modulation(M) mode
Timer Switch
Set 30 minutes, 60 minutes, or Constant .
Frequency Control
Adjust Frequency from 2 Hz to 150 Hz by turning the control
Pulse Width Control
Adjust Pulse Width from 60 µs to 250 µs by turning the control
Battery compartment
9 Voltage battery- 1 pc
Lid Cover
A panel covers the controls for Mode, Timer, Frequency & Pulse WIdth. Your medical professional may ask to set
these controls for you and request that you leave the cover in place.
Amplitude Controls
It controls the "INTENSITY" level of stimulating pulses. These controls located at the top of the unit regulate the
amplitude, or intensity, of the stimulation and are the ON/OFF CONTROL. The power indicator will light up with
green color when the unit is working.
Caution: If the stimulation levels are uncomfortable or become uncomfortable, reduce the stimulation intensity to a comfortable level
and contact your physician if problems persist.

56
Mode Switch
Set Normal (N), Burst(B), or Modulation(M) mode
Timer Switch
Set 30 minutes, 60 minutes, or Constant .
Frequency Control
Adjust Frequency from 2 Hz to 150 Hz by turning the control
Pulse Width Control
Adjust Pulse Width from 60 µs to 250 µs by turning the control
Battery compartment
9 Voltage battery- 1 pc
Lid Cover
A panel covers the controls for Mode, Timer, Frequency & Pulse WIdth. Your medical professional may ask to set
these controls for you and request that you leave the cover in place.
Amplitude Controls
It controls the "INTENSITY" level of stimulating pulses. These controls located at the top of the unit regulate the
amplitude, or intensity, of the stimulation and are the ON/OFF CONTROL. The power indicator will light up with
green color when the unit is working.
Caution: If the stimulation levels are uncomfortable or become uncomfortable, reduce the stimulation intensity to a comfortable level
and contact your physician if problems persist.

3.2 NORMAL Mode:
The Normal mode produces a continuous train of impulses. The stimulation
parameters are not automatically interrupted nor varied in any way. In this mode, the
pulse rate (from 2 to 150Hz) and pulse width (from 60 to 250µs) are fully adjustable.
The normal mode is quite versatile because it may be applied with a variety of rate
and width settings.
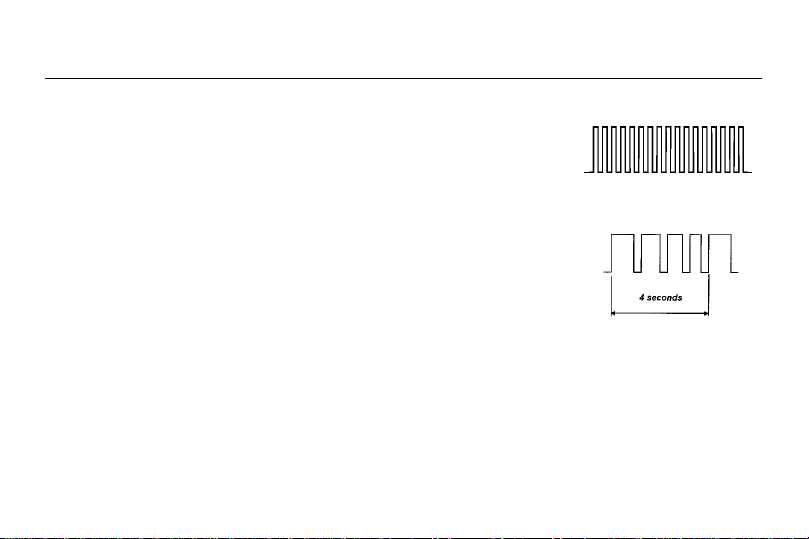
3.3 Modulation Mode:
Pulse width shows decrease/ increase cycle of variation. For example, if pulse width is
set for the maximum output value (250µs), pulse width will decrease from 250µs to
125µs and then increase back to 250µs again in period of 4 seconds.
The mode switch offers three stimulation modes. The mode switch is located
under the front lid cover and you can shift the "MODE" switch to adjust the
mode.
Be sure that when adjusting these stimulation modes, the intensity (Amplitude)
output controls are set to the minimum output positions.
3.1 BURST Mode:
The burst mode provides a "burst" of seven pulses. There are two bursts
delivered per second.
3.STIMULATION MODES
8
7
3.2 NORMAL Mode:
The Normal mode produces a continuous train of impulses. The stimulation
parameters are not automatically interrupted nor varied in any way. In this mode, the
pulse rate (from 2 to 150Hz) and pulse width (from 60 to 250µs) are fully adjustable.
The normal mode is quite versatile because it may be applied with a variety of rate
and width settings.
3.3 Modulation Mode:
Pulse width shows decrease/ increase cycle of variation. For example, if pulse width is
set for the maximum output value (250µs), pulse width will decrease from 250µs to
125µs and then increase back to 250µs again in period of 4 seconds.
The mode switch offers three stimulation modes. The mode switch is located
under the front lid cover and you can shift the "MODE" switch to adjust the
mode.
Be sure that when adjusting these stimulation modes, the intensity (Amplitude)
output controls are set to the minimum output positions.
3.1 BURST Mode:
The burst mode provides a "burst" of seven pulses. There are two bursts
delivered per second.
3.STIMULATION MODES
8
7
9
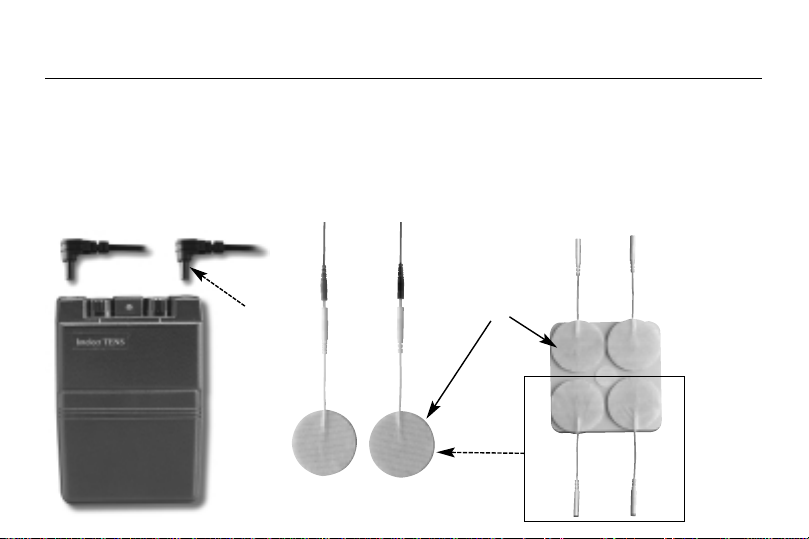
4.3 Connect lead wires to unit:
Before proceeding to this step, be sure the unit is completely turned OFF. Holding the insulated portion of the lead
wire connector, insert the angled-"L" plug into the receptacle on the top of the main unit. Ensure the lead wires are
inserted correctly. The unit has two output receptacles controlled by Channel 1 and Channel 2 Amplitude Control
knobs at the top of the unit. You may choose to use one channel with one pair of lead wires or both channels with
two pairs of lead wires. Using both channels gives the user the advantage of stimulating two different areas at the
same time.
NOTE: Always read this instruction manual before use.
PREPARATION FOR USE
4.1 Check Battery:
Insert a fresh 9V alkaline or rechargeable battery into the battery compartment. Make
sure you are installing the battery properly. The battery is inserted in the casing on the
foot of the stimulator unit.
BE SURE TO MATCH THE POSITIVE AND NEGATIVE ENDS OF THE BATTERY TO
THE MARKINGS IN THE BATTERY COMPARTMENT OF UNIT.
CONNECTING THE STIMULATOR
4.2 Connect electrodes to lead wires:
Insert the lead wire connector into electrodes connector (standard 0.08 inch female
connection). MAKE SURE NO BARE METAL OF THE PINS IS EXPOSED
Caution:
Always use the electrodes with the requirements of the EN60601-1 and EN60601-2, such as with
CE mark, or which are legally marketed in the US under 510(K) procedure.
10
4. INSTRUCTIONS FOR USE
Self-adhesive
Electrodes Pads
Angle"L"-shape
plug
9
4.3 Connect lead wires to unit:
Before proceeding to this step, be sure the unit is completely turned OFF. Holding the insulated portion of the lead
wire connector, insert the angled-"L" plug into the receptacle on the top of the main unit. Ensure the lead wires are
inserted correctly. The unit has two output receptacles controlled by Channel 1 and Channel 2 Amplitude Control
knobs at the top of the unit. You may choose to use one channel with one pair of lead wires or both channels with
two pairs of lead wires. Using both channels gives the user the advantage of stimulating two different areas at the
same time.
NOTE: Always read this instruction manual before use.
PREPARATION FOR USE
4.1 Check Battery:
Insert a fresh 9V alkaline or rechargeable battery into the battery compartment. Make
sure you are installing the battery properly. The battery is inserted in the casing on the
foot of the stimulator unit.
BE SURE TO MATCH THE POSITIVE AND NEGATIVE ENDS OF THE BATTERY TO
THE MARKINGS IN THE BATTERY COMPARTMENT OF UNIT.
CONNECTING THE STIMULATOR
4.2 Connect electrodes to lead wires:
Insert the lead wire connector into electrodes connector (standard 0.08 inch female
connection). MAKE SURE NO BARE METAL OF THE PINS IS EXPOSED
Caution:
Always use the electrodes with the requirements of the EN60601-1 and EN60601-2, such as with
CE mark, or which are legally marketed in the US under 510(K) procedure.
10
4. INSTRUCTIONS FOR USE
Self-adhesive
Electrodes Pads
Angle"L"-shape
plug

4.7 Adjust the Frequency:
The frequency is adjustable 2~150Hz.
Turn Frequency Control to adjust Frequency to the setting recommended by your medical
professional.
4.8 Adjust Timer:
The timer is adjustable: 30 minutes, 60 minutes, or Constant.
Shift TIMER switch to set the stimulation time recommended by your physician or therapist.
4.9 Adjust Channel Amplitude:
Turn Channel 1 or 2 clockwise. Slowly turn the Channel control until you reach the setting
recommended by your medical professional. Repeat for the other channel, if both channels
are to be used.
Caution: If the stimulation levels are uncomfortable or become uncomfortable, reduce the stimulation
intensity to a comfortable level and contact your medical practitioner if problems persist.
4.4 Place electrodes on skin:
Apply electrodes to the exact site indicated by your physician, following the instruction
included with the electrodes labeling. Before applying electrodes, be sure the skin surface
over which electrodes are placed is thoroughly cleaned and dried. Make sure the
electrodes are placed firmly to the skin and make good contact between the skin and the
electrodes. Place the electrodes over the skin; attach them properly, firmly, and evenly.
ADJUSTING THE CONTROLS
4.5 Select the mode:
Shift "MODE" switch to set the stimulation mode recommended by your physician or
therapist. For details about stimulating waveform and sequences, please refer to Sec. 3
"Stimulation Modes".
Caution: Consult your physician for your suitable stimulation mode.
4.6 Adjust the Pulse Width:
The pulse width is adjustable 60~250µs.
Turn Pulse Width Control to adjust pulse width to the setting recommended by your
medical professional.
12
11

4.7 Adjust the Frequency:
The frequency is adjustable 2~150Hz.
Turn Frequency Control to adjust Frequency to the setting recommended by your medical
professional.
4.8 Adjust Timer:
The timer is adjustable: 30 minutes, 60 minutes, or Constant.
Shift TIMER switch to set the stimulation time recommended by your physician or therapist.
4.9 Adjust Channel Amplitude:
Turn Channel 1 or 2 clockwise. Slowly turn the Channel control until you reach the setting
recommended by your medical professional. Repeat for the other channel, if both channels
are to be used.
Caution: If the stimulation levels are uncomfortable or become uncomfortable, reduce the stimulation
intensity to a comfortable level and contact your medical practitioner if problems persist.
4.4 Place electrodes on skin:
Apply electrodes to the exact site indicated by your physician, following the instruction
included with the electrodes labeling. Before applying electrodes, be sure the skin surface
over which electrodes are placed is thoroughly cleaned and dried. Make sure the
electrodes are placed firmly to the skin and make good contact between the skin and the
electrodes. Place the electrodes over the skin; attach them properly, firmly, and evenly.
ADJUSTING THE CONTROLS
4.5 Select the mode:
Shift "MODE" switch to set the stimulation mode recommended by your physician or
therapist. For details about stimulating waveform and sequences, please refer to Sec. 3
"Stimulation Modes".
Caution: Consult your physician for your suitable stimulation mode.
4.6 Adjust the Pulse Width:
The pulse width is adjustable 60~250µs.
Turn Pulse Width Control to adjust pulse width to the setting recommended by your
medical professional.
12
11

14
4.13 Care of Electrodes:
To avoid skin irritation and ensure good contact with skin, clean silicone rubber electrodes
with soap and water frequently. The electrodes must be dried completely before using.
✽
If you are using self-adhesive electrodes, disregard this procedure.
✽
Always use the electrodes with the requirements of the EN60601-1 and EN60601-2, such as with
CE
mark, or which are legally marketed in the US under 510(K) procedure.
4.14 Care of Electrode cords:
Clean the electrode cords by wiping them with damp cloth. Coating them lightly with
talcum powder will reduce tangles and prolong the life.
4.10 Turn Unit Off:
Turn both Channel Amplitude controls to off. Unplug the electrode lead wires, grasping
them by the plug, not the cord. If treatment will be resumed shortly, the electrodes may be
left on the skin. When the electrodes are removed, clean the skin thoroughly with mild
soap and water. If there is skin irritation, consult your medical professional.
CARE AND MAINTENANCE
4.11 Portability:
Your unit is portable and may be clipped to a belt, shirt pocket, bra, or other clothing.
4.12 Battery:
To replace the battery, open the lid cover and extract the battery. Replace it with a 9 V
alkaline or similar rechargeable battery. Make sure you insert the battery correctly.
13

14
4.13 Care of Electrodes:
To avoid skin irritation and ensure good contact with skin, clean silicone rubber electrodes
with soap and water frequently. The electrodes must be dried completely before using.
✽
If you are using self-adhesive electrodes, disregard this procedure.
✽
Always use the electrodes with the requirements of the EN60601-1 and EN60601-2, such as with
CE
mark, or which are legally marketed in the US under 510(K) procedure.
4.14 Care of Electrode cords:
Clean the electrode cords by wiping them with damp cloth. Coating them lightly with
talcum powder will reduce tangles and prolong the life.
4.10 Turn Unit Off:
Turn both Channel Amplitude controls to off. Unplug the electrode lead wires, grasping
them by the plug, not the cord. If treatment will be resumed shortly, the electrodes may be
left on the skin. When the electrodes are removed, clean the skin thoroughly with mild
soap and water. If there is skin irritation, consult your medical professional.
CARE AND MAINTENANCE
4.11 Portability:
Your unit is portable and may be clipped to a belt, shirt pocket, bra, or other clothing.
4.12 Battery:
To replace the battery, open the lid cover and extract the battery. Replace it with a 9 V
alkaline or similar rechargeable battery. Make sure you insert the battery correctly.
13

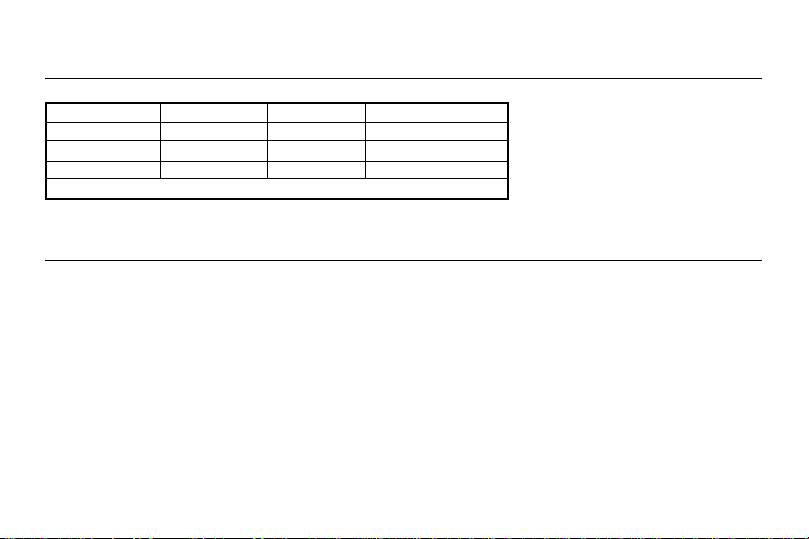
Channel Dual channels, isolated between channels
Pulse Amplitude 0 ~ 80 mA = 0 ~ 40 volts, adjustable (at 500 ohm load)
Pulse Frequency (Hz) 2 ~ 150
Pulse Width (µs) 60 ~ 250
Waveform Asymmetric biphasic square pulse.
Timer Control (mins) 30, 60, or Constant
Power Supply 9V DC square shape battery
Size (D x W x H) 1.0" x 2.4" x 3.6" (26 mm x 62 mm x 91 mm)
Weight (including battery) 4.4 oz (126 g)
Safety standard EN 60601-1, EN 60601-1-2, IEC 60601-2-10
Operation Ambient Temperature Range 50 ~ 95ºF (10 ~ 35ºC)
Operation Ambient Humidity Range 20 ~ 90% RH
Storage & Transportation Temperature Range 32 ~ 158º F (0 ~ 70ºC)
Storage & Transportation Humidity Range 20 ~ 90% RH
All values have ±10% tolerance.
16
15
6. SPECIFICATION
5. HANDLING AND STORAGE
Keep this device in the carrying case and store it at room temperature. Stimulation Modes descriptions
Mode BURST NORMAL Modulation
Frequency 2~150Hz 2~150Hz, 2~150Hz
Pulse Width 60~250µs 60~250µs 60~250µs
Cycle Time 0.5 Sec. Constant. 4 Secs.
Loading: 500Ω
*All values have±10% tolerance.
7 ACCESSORIES
Self-Adhesive Electrodes 4 PCS.
9 V Battery 1 PC.
Lead Wires 2 PCS.
Instruction Manual 1 PC.
Channel Dual channels, isolated between channels
Pulse Amplitude 0 ~ 80 mA = 0 ~ 40 volts, adjustable (at 500 ohm load)
Pulse Frequency (Hz) 2 ~ 150
Pulse Width (µs) 60 ~ 250
Waveform Asymmetric biphasic square pulse.
Timer Control (mins) 30, 60, or Constant
Power Supply 9V DC square shape battery
Size (D x W x H) 1.0" x 2.4" x 3.6" (26 mm x 62 mm x 91 mm)
Weight (including battery) 4.4 oz (126 g)
Safety standard EN 60601-1, EN 60601-1-2, IEC 60601-2-10
Operation Ambient Temperature Range 50 ~ 95ºF (10 ~ 35ºC)
Operation Ambient Humidity Range 20 ~ 90% RH
Storage & Transportation Temperature Range 32 ~ 158º F (0 ~ 70ºC)
Storage & Transportation Humidity Range 20 ~ 90% RH
All values have ±10% tolerance.
16
15
6. SPECIFICATION
5. HANDLING AND STORAGE
Keep this device in the carrying case and store it at room temperature. Stimulation Modes descriptions
Mode BURST NORMAL Modulation
Frequency 2~150Hz 2~150Hz, 2~150Hz
Pulse Width 60~250µs 60~250µs 60~250µs
Cycle Time 0.5 Sec. Constant. 4 Secs.
Loading: 500Ω
*All values have±10% tolerance.
7 ACCESSORIES
Self-Adhesive Electrodes 4 PCS.
9 V Battery 1 PC.
Lead Wires 2 PCS.
Instruction Manual 1 PC.

8. TROUBLESHOOTING 9. WARRANTY
18
17
•The power indicator lights up but
unit does not function properly. •"On" and "Battery Light" are dim. •None of indicators lights up.
1. Check all control settings. Are
they set to values prescribed by
your medical professional?
2. Are electrodes in proper position?
3. Check lead wires. Be sure all
connectors are firmly sealed.
4. Replace cord set with another to
check for broken wires.
1. Replace battery with a new one. 1. Replace battery with a new one.
If your unit does not seem to operate correctly, refer to the chart below to determine what may
be wrong. If none of these measures correct the problem, the unit should be serviced. * Unit: One year (12 months) from the date of the original consumer purchase.
* Accessories (consisting of lead wire, AC adapter, electrodes, carrying case, and belt clip): 90 days from the date of
original consumer purchase.
To obtain service from Chattanooga Group or the selling dealer under this warranty, a written claim must be made
within the warranty period to Chattanooga Group or the selling dealer.
Chattanooga Group shall not be held liable in any event for incidental or consequential damages. Some states do not
allow exclusion or limitation of incidental or consequential damages so the above limitation or exclusion may not
apply to you
Table of contents
Other Intelect Medical Equipment manuals