IMPORTANT INFORMATION
The iReliev®Back Pain Relief System is particularly safe and user-
friendly. When the Wrap is fastened and the electrode pads are in
contact with the skin, the intensity of the iReliev®TENS device may
be adjusted.
iReliev®Back Pain Relief System
Model # ET-9090
Table of Contents
2
Introduction ................................................................................................................................................................... 3
Safety Information .............................................................................................................................................. 4
Battery Safety Information ................................................................................................................. 5
Cautions & Contraindications ....................................................................................................... 5
Package Contents ............................................................................................................................................... 6
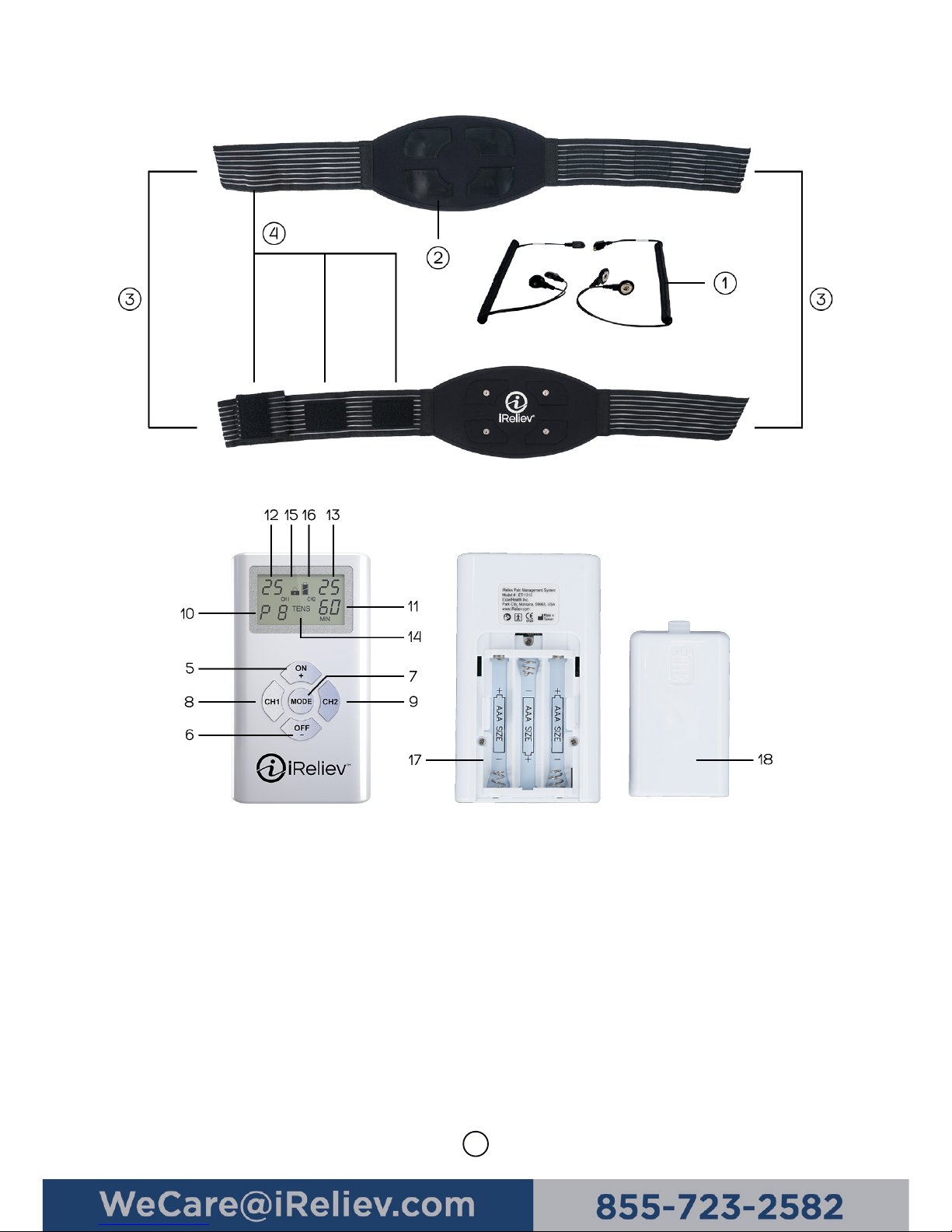
Parts Diagram/Features ............................................................................................................................. 7
Inserting/Changing the Batteries ................................................................................................ 8
Putting on the Wrap ........................................................................................................................................ 9
Turning on the Device .................................................................................................................................. 10
Turning off the Device ................................................................................................................................. 10
Selecting Treatment Time .................................................................................................................. 11
Selecting the Program ............................................................................................................................... 11
Selecting the Therapy Intensity Level .............................................................................. 11
Special Features .................................................................................................................................................... 11
Care & Maintenance ........................................................................................................................................ 12
Disposal ................................................................................................................................................................................ 12
Troubleshooting ..................................................................................................................................................... 13
Technical Specifications .................................................................................................................. 14 -18
Warranty ............................................................................................................................................................................. 19
Contact ................................................................................................................................................................................. 19