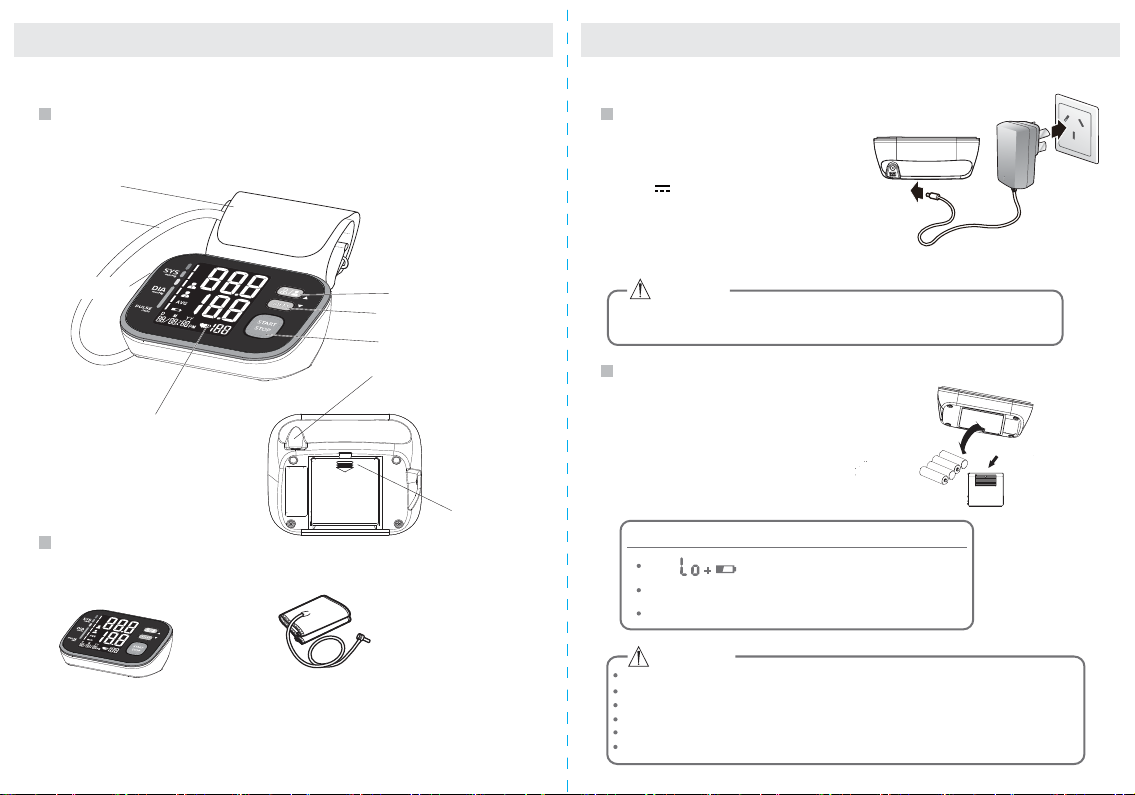
INTRODUCTIONINTRODUCTION
4 5
CAUTION
* Do not take any therapeutic measures on the basis of a self measurement. Never alter the dose of a
medicine prescribed by a doctor. Consult your doctor if you have any question about your blood pressure.
* When the device was used to measure patients who have common arrhythmias such as atrial or
ventricular premature beats or atrial fibrillation, the best result may occur with deviation. Please consult
your physician about the result.
* Don't kink the connection tube during use, otherwise, the cuff pressure may continuously increase
which can prevent blood flow and result in harmful injury to the PATIENT.
CAUTION
* This device is intended for adult use in homes only.
* The device is not suitable for use on neonatal patients, pregnant women,patients with implanted,
electronical devices, patients with pre-eclampsia, premature ventricular beats, atrial fibrillation,
peripheral, arterial disease and patients undergoing intravascular therapy or arterio-venous shunt or
people who received a mastectomy. Please consult your doctor prior to using the unit if you suffer from
illnesses.
* The device is not suitable for measuring the blood pressure of children. Ask your doctor before using it
on older children.
* The device is not intended for patient transport outside a healthcare facility.
* The device is not intended for public use.
* This device is intended for no-invasive measuring and monitoring of arterial blood pressure.It is not
intended for use on extremities other than the arm or for functions other than obtaining a blood pressure
measurement.
* Do not confuse self-monitoring with self-diagnosis. This unit allows you to monitor your blood
pressure.Do not begin or end medical treatment without asking a physician for treatment advice.
* If you are taking medication,consult your physician to determine the most appropriate time to measure
your blood pressure. Never change a prescribed medication without consulting your physician.
The signs below might be in the user manual, labeling or other component.
They are the requirement of standard and using.
Safety Information
* When using this device, please pay attention to the following situation which may interrupt blood flow and
influence blood circulation of the patient, thus cause harmful injury to the patient: connection tubing kinking
too frequent and consecutive multiple measurements; the application of the cuff and its pressurization on
any arm where intravascular access or therapy, or an arterio-venous (A-V) shunt, is present; inflating the
cuff on the side of a mastectomy.
* Warning: Do not apply the cuff over a wound;otherwise it can cause further injury.
*Do not inflate the cuff on the same limb which other monitoring ME equipment is applied around
simultaneously, because this could cause temporary loss of function of those simultaneously-used
monitoring ME equipment.
*On the rare occasion of a fault causing the cuff to remain fully inflated during measurement, open the cuff
immediately. Prolonged high pressure (cuff pressure >300mmHg or constant pressure >15mmHg for
more than 3 minutes) applied to the arm may lead to an ecchymosis.
*Please check that operation of the device does not result in prolonged impairment of patient blood
circulation.
* When measurement, please avoid compression or restriction of the connection tubing.
* The device cannot be used with HF surgical equipment at the same time.
* The ACCOMPANYING DOCUMENT shall disclose that the SPHYGMOMANOMETER was clinically
investigated according to the requirements of ISO 81060-2:2013.
* To verify the calibration of the AUTOMATED SPHYGMOMANOMETER, please contact the manufacturer.
* This device is contraindicated for any female who may be suspected of, or is pregnant. Besides providing
inaccurate readings, the effects of this device on the fetus are unknown.
* Too frequent and consecutive measurements could cause disturbances in blood circulation and injuries.
* This unit is not suitable for continuous monitoring during medical emergencies or operations.Otherwise,
the patient’s arm and fingers will become anaesthetic, swollen and even purple due to a lack of blood.
* When not in use, store the device with the adapter in a dry room and protect it against extreme moisture,
heat, lint, dust and direct sunlight. Never place any heavy objects on the storage case.
* This device may be used only for the purpose described in this booklet. The manufacturer cannot be held
liable for damage caused by incorrect application.
*This device comprises sensitive components and must be treated with caution. Observe the storage and
operating conditions described in this booklet.
* The equipment is not AP/APG equipment and not suitable for use in the presence of a flammable
anesthetic mixture with air of with oxygen or nitrous oxide.
* Warning: No servicing/maintenance while the ME equipment is in use.
* The patient is an intended operator.
* The patient can measure data and change battery under normal circumstances and maintain the device
and its accessories according to the user manual.
* To avoid measurement errors, please avoid the condition of strong electromagnetic field radiated
interference signal or electrical fast transient/burst signal.
* The blood pressure monitor, its adaptor, and the cuff are suitable for use within the patient environment.
If you are allergic to polyester, nylon or plastic, please don't use this device.
* During use, the patient will be in contact with the cuff. The materials of the cuff have been tested and
found to comply with requirements of ISO 10993-5:2009 and ISO 10993-10:2010. It will not cause any
potential sensization or irritation reaction.
* Adaptor is specified as a part of ME EQUIPMENT.
Classification for water ingress
and particulate matter
Read the instructions (actual
symbol colours are white on a
blue background).
Symbol for “MANUFACTURER”
Symbol for “SERIAL NUMBER”
Symbol for “TYPE BF APPLIED
PARTS”
Symbol for “DIRECT CURRENT”
Symbol for “RECYCLE”
Symbol for “ENVIRONMENT
PROTECTION - Electrical waste
products should not be disposed of
with household waste. Please recycle
where facilities exist. Check with your
local authority or retailer for recycling
advice”
Symbol for “Authorised Representative
in the European Community”
EC REP
Symbol for “MANUFACTURE
DATE”
Caution: These notes must be
observed to prevent any damage
to the device.
SN
0197
Symbol for “COMPLIES WITH
MDD 93/42/EEC
REQUIREMENTS”