14 15
MARA OPERATOR’S MANUAL MARA OPERATOR’S MANUAL
⚠Precaution: Console requires hospital grade power supply 110 to 120 V,
60 Hz with a minimum of 15 amp service. Hospital grade receptacles
may be marked with a green dot, or wording such as “Hospital Grade” or
“Hosp. Grade.” Consult your institution’s biomedical department if unsure.
⚠Warning: The use of accessories, transducers and cables, other than
those specified by AEGEA Medical Inc., may result in increased emissions
or decreased immunity of the Console. Do not use accessories,
transducers and cables other than those specified.
4. Insert the power cord to a grounded wall receptacle.
⚠Warning: Connect the Console power cord to a properly grounded
receptacle. Do not use power plug adapters. Do not use extension cords.
⚠Warning: Ensure that the Console is connected to a reliable power
source. Unreliable or inconsistent power may cause power loss to the
Console resulting in an interruption of the procedure.
⚠Warning: To avoid the risk of electric shock, this equipment must only
be connected to a supply mains with protective earth.
⚠Precaution: A temporary circuit overload can cause the internal circuit
breaker to trip. If this were to occur, the power should be turned off, the
Circuit Breaker reset switch should be pressed back in, and then the
unit can be powered back on.
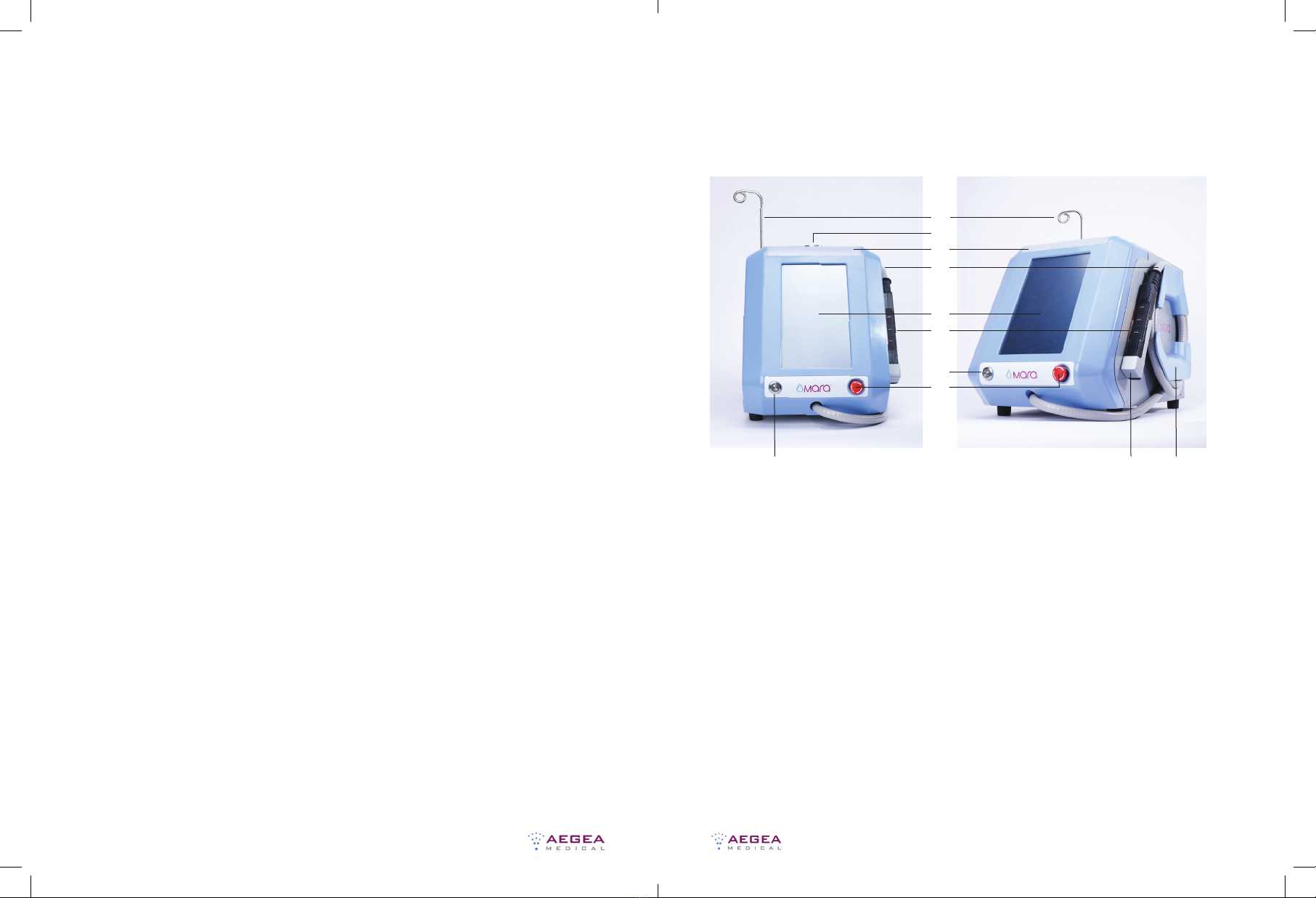
5. Verify that the red Interrupt Button on the front panel is not engaged
(twist to disengage).
6. Press the Front Panel Power Button to turn on the Console, and verify
the following:
a. The blue light on the power button illuminates when the power is on
b. The light above the display temporarily illuminates
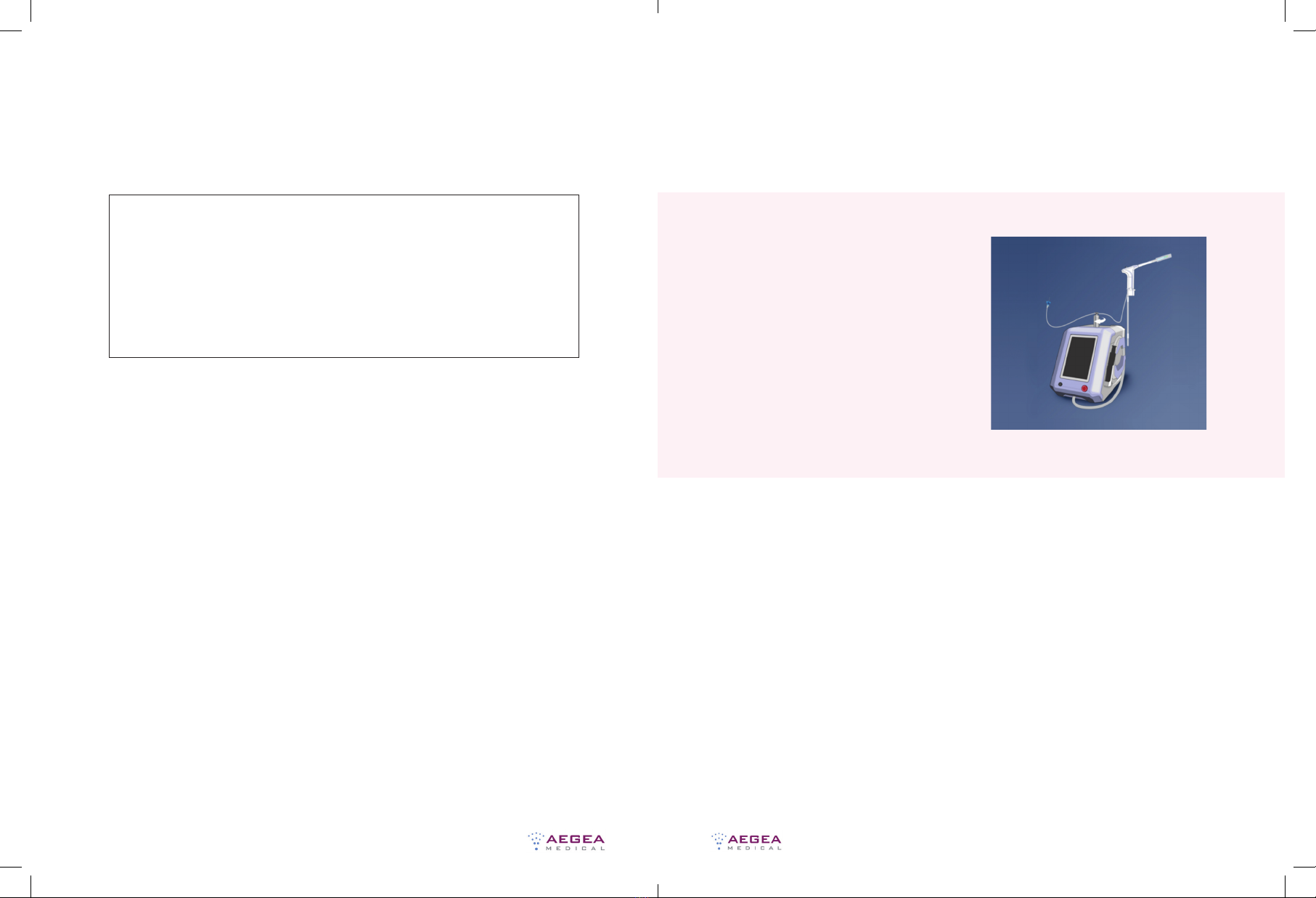
c. The display turns on and temporarily flashes an AEGEA logo ( i m ag e 1)
d. An activation tone sounds indicating the speaker is functional
e. The Console self-test (power on self-test) successfully completes
(a status bar will show progression of the power on self-test)
Setup Instructions
• If the Console was transported or stored at temperatures outside the
Transport and Storage ambient temperature range, allow at least
one hour for the Console to reach room temperature before use. Refer
to Appendix A for more information.
• If the Console was stored without use for longer than one year, the
Console should be tested and calibrated before use.
Setting up the Mara Console for the first time:
1. Unbox the Console. Place the Console on a sturdy cart or table with a
stable flat surface to the left or right side of the user. The front panel
should be facing the user such that there is an unobstructed view of
the touchscreen.
2. With the Console on a cart or table, ensure there are at least four to six
inches of space from the sides and top of the Console to ensure proper
airflow for cooling. It is normal for the outer surfaces of the Console to
be warm when in use.
⚠Precaution: The Console should not be stacked or used adjacent to
other electrical equipment. Do not stack equipment to block the vents
on the bottom or sides of the Console. If stacked or adjacent use is
necessary, the Console should be observed to verify normal operation.
⚠Precaution: Provide as much distance as possible between the Console
and other electrical equipment to prevent interference. Portable and
mobile RF communications equipment can affect the Console.
⚠Warning: Portable RF communications equipment (including
peripherals such as antenna cables and external antennas) should be
used no closer than 30 cm (12 inches) to any part of the Mara Water
Vapor Ablation System, including cables specified by AEGEA Medical
Inc. Otherwise, degradation of the performance of this equipment
could result.
3. Attach the power cord provided with the Console to the Console’s
rear panel receptacle.