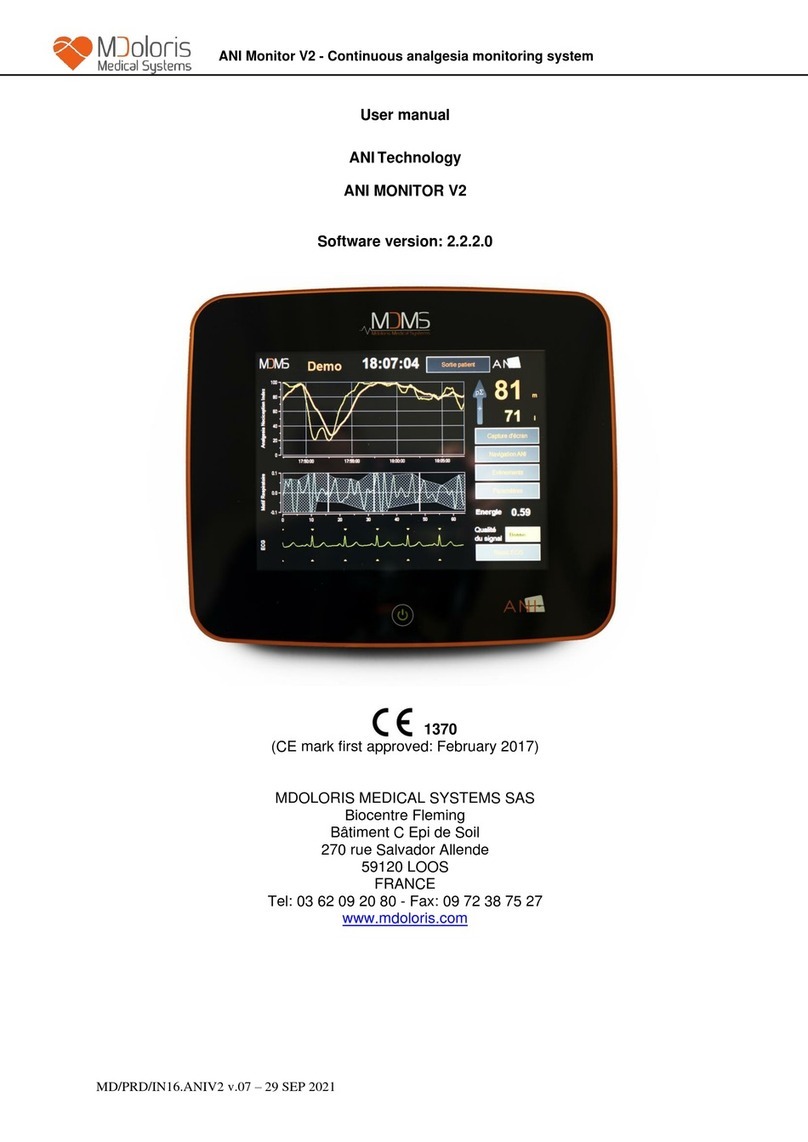
ANI Monitor V1 - Continuous analgesia monitoring system
MD/PRD/IN16.ANIV1 V.12 - 08 MAR 2019
Contents
1Safety precautions .........................................................................................................1
1.1 Warning....................................................................................................................1
1.2 Caution.....................................................................................................................6
1.3 Notes ........................................................................................................................8
1.4 Key to symbols.........................................................................................................8
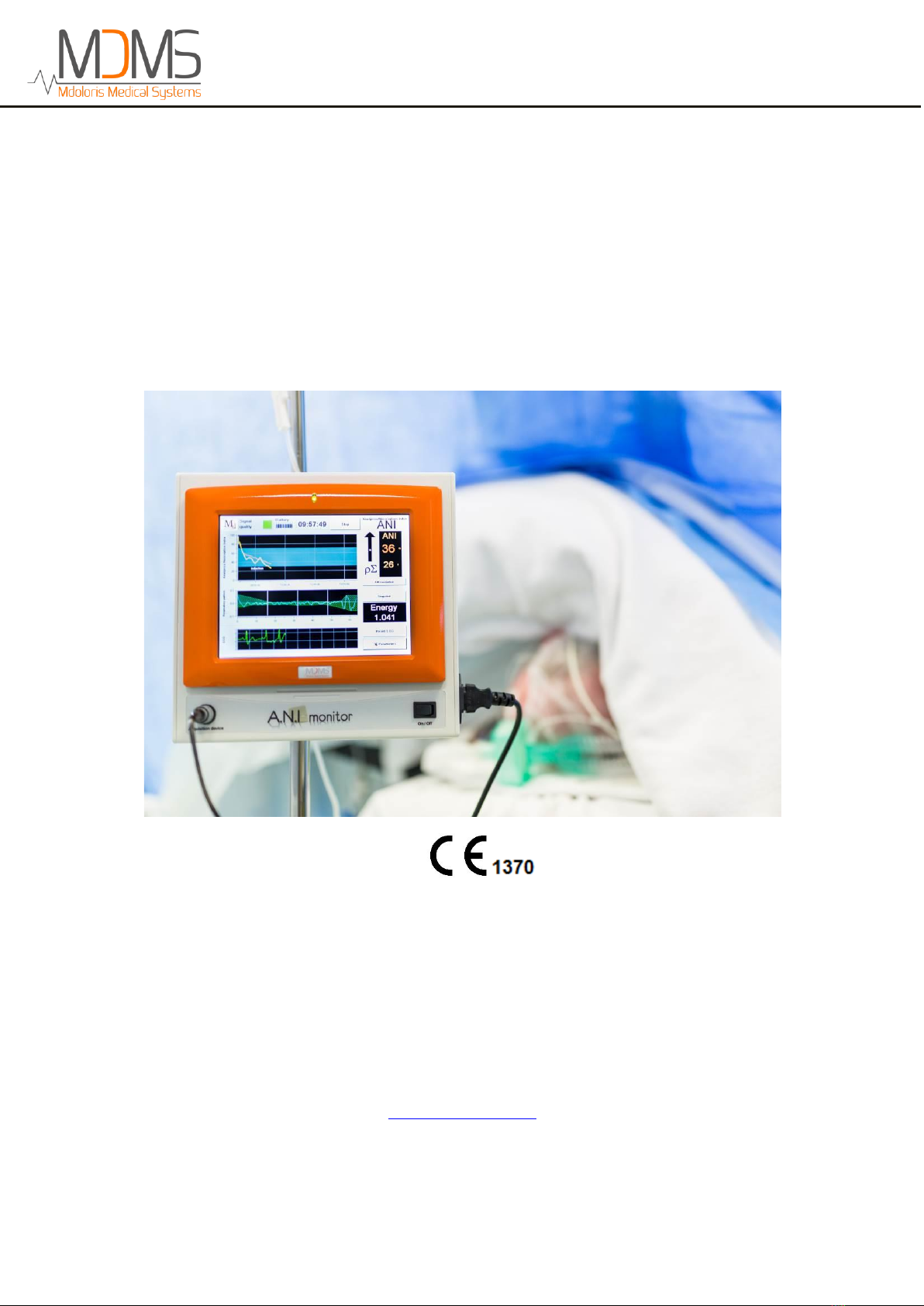
2ANI Monitor V1 ............................................................................................................9
3Installing the ANI Monitor V1....................................................................................11
3.1 Perfusion stand .......................................................................................................11
3.2 ANI Sensor V1 / ANI Sensor V2 / ANI Sensor V1 PLUS .......................................11
3.3 ANI Monitor V1 connection ...................................................................................13
3.4 Battery....................................................................................................................14
4Beginning ANI Monitoring .........................................................................................15
5Using the ANI Monitor V1 and setting parameters ...................................................17
5.1 ECG Capture ..........................................................................................................17
5.2 Respiratory pattern..................................................................................................18
5.3 ANI index...............................................................................................................19
5.4 ANI navigation.......................................................................................................19
6ANI Monitor V1 settings.............................................................................................20
6.1 Language parameters ..............................................................................................20
6.2 Threshold................................................................................................................20
6.3 Events.....................................................................................................................23
6.4 Expert mode and Energy index ...............................................................................23
7Ending ANI monitoring ..............................................................................................26
7.1 Stopping the recording of a case..............................................................................26
7.2 Demo......................................................................................................................27
7.3 New patient.............................................................................................................27
7.4 Maintenance ...........................................................................................................27
7.5 Deleting patient data...............................................................................................27
7.6 Screen capture ........................................................................................................28
7.7 Exporting data files.................................................................................................29
7.8 Updating events......................................................................................................30
7.9 Setting date and time...............................................................................................31
7.10 Updating monitor....................................................................................................32
7.11 Shutting down.........................................................................................................34
8Trouble shooting..........................................................................................................34