Table of contents
1 Safety precautions .............................................................................................................. 5
1.1 Warnings...................................................................................................................... 5
1.2 Cautions..................................................................................................................... 10
1.3 Notes.......................................................................................................................... 12
1.4 Key to Symbols ......................................................................................................... 12
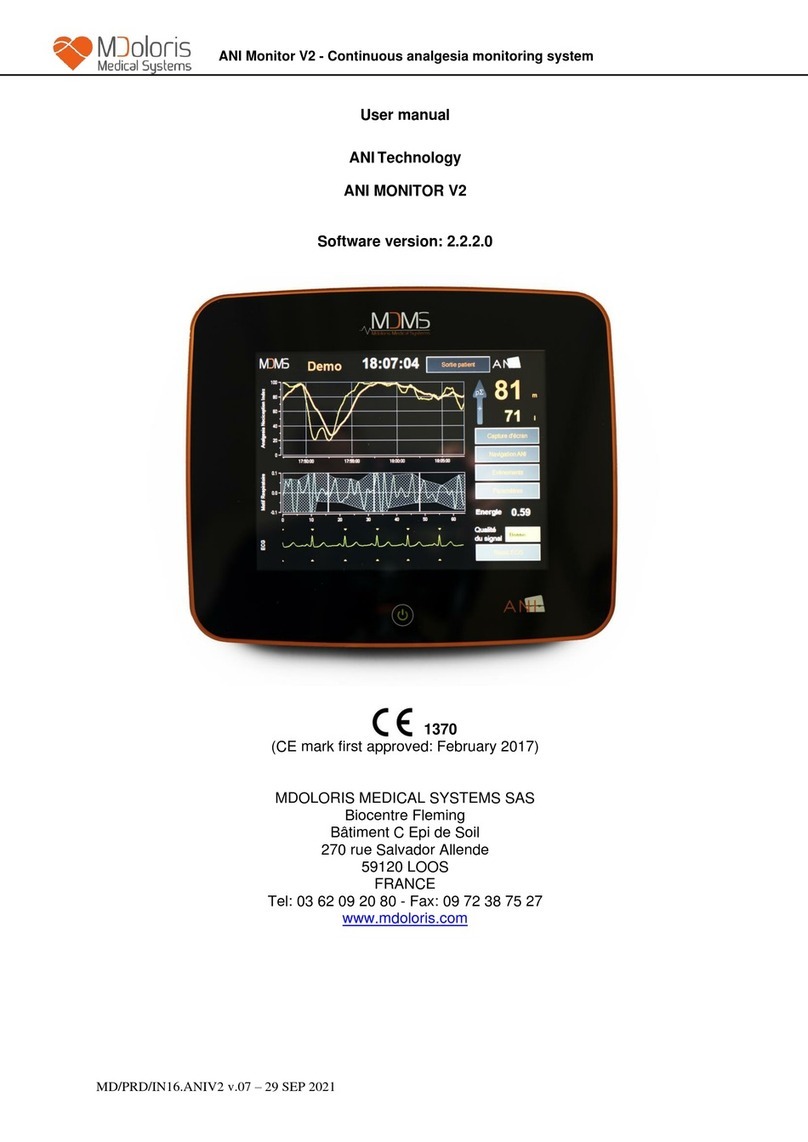
2ANI monitor presentation.............................................................................................. 13
3ANI monitor installation ................................................................................................ 14
3.1 Clamping support ...................................................................................................... 14
3.2 Skin electrodes...............................................................................................................
3.3 ANI monitor connexion............................................................................................. 17
3.4 Battery ....................................................................................................................... 18
4Beginning the ANI monitoring ......................................................................................19
5ANI computing process .................................................................................................. 22
5.1 ECG Capture.............................................................................................................. 22
5.2 Respiratory Pattern .................................................................................................... 22
5.3 ANI Index.................................................................................................................. 23
5.4 Navigation panel........................................................................................................ 24
6ANI monitor setup .......................................................................................................... 25
6.1 Threshold................................................................................................................... 25
6.2 Events insert .............................................................................................................. 28
6.3 Expert mode and Energy index ................................................................................. 29
7Operating the ANI monitor............................................................................................ 32
7.1 Quit Patient................................................................................................................ 32
7.2 Demo ......................................................................................................................... 33
7.3 New patient................................................................................................................ 33
7.4 Maintenance............................................................................................................... 33
7.5 Delete patients data.................................................................................................... 34
7.6 Screen shot................................................................................................................. 35
7.7 Export Data................................................................................................................ 35
7.8 Update of events........................................................................................................ 37
7.9 Date and time settings................................................................................................ 38
7.10 Update monitor.......................................................................................................... 38
7.11 Shutting down the ANI monitor................................................................................ 41
8Troubleshooting .............................................................................................................. 41
9Monitor disposal.............................................................................................................. 42
10 Environnement................................................................................................................ 43
10.1 Shipping and Storage Environnement....................................................................... 43
10.2 Operating Environnement.......................................................................................... 43
10.3 Power Requirements and System Grounding............................................................ 44
11 Care, cleaning and maintenance.................................................................................... 44