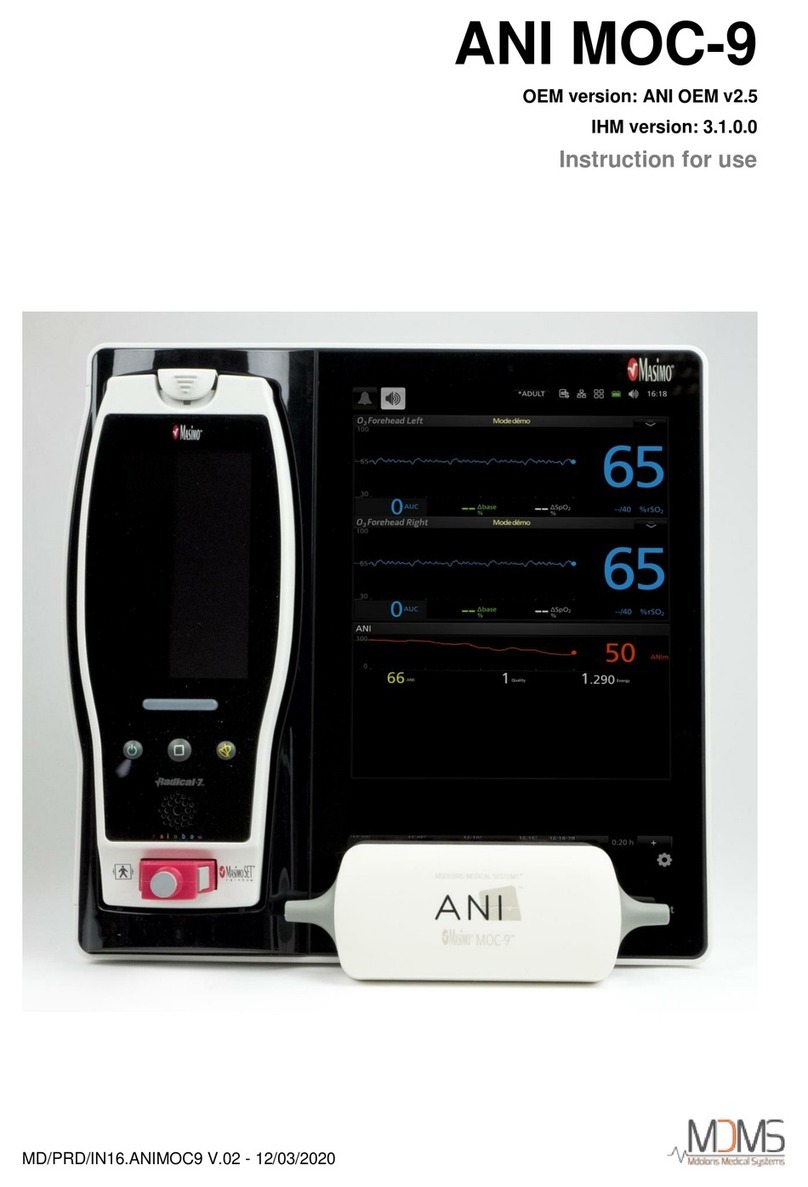
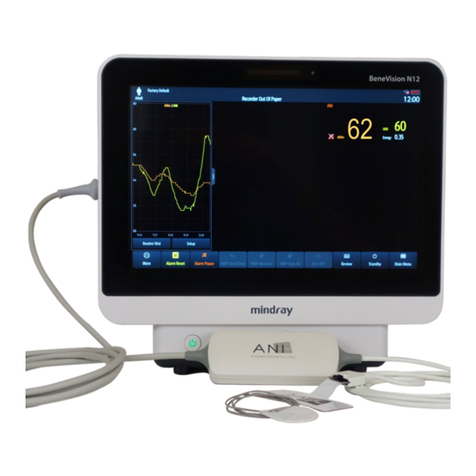
ANI Monitor V2 - Continuous analgesia monitoring system
MD/PRD/IN16.ANIV2 v.07 –29 SEP 2021 P a g e 3 | 44
Contents
1Safety precautions......................................................................................................... 5
1.1 Warnings and safety instructions............................................................................ 5
1.2 Key to symbols......................................................................................................... 10
2ANI MONITOR V2.......................................................................................................... 11
3Installating the ANI MONITOR V2 ............................................................................ 11
3.1 Perfusion stand........................................................................................................ 11
3.2 Table.......................................................................................................................... 11
3.3 ANI-SENS-V1........................................................................................................... 12
3.4 ANI MONITOR V2 Connection.............................................................................. 13
4Beginning ANI monitoring V2................................................................................... 14
5Using the ANI MONITOR V2 and setting parameters......................................... 16
5.1 ECG Capture............................................................................................................ 16
5.2 Respiratory pattern.................................................................................................. 17
5.3 ANI index.................................................................................................................. 18
5.4 ANI navigation.......................................................................................................... 19
6ANI MONITOR V2 settings......................................................................................... 19
6.1 Language parameters............................................................................................. 19
6.2 Threshold.................................................................................................................. 20
6.3 Events ....................................................................................................................... 22
6.4 Expert mode and energy index............................................................................. 23
7Ending ANI monitoring............................................................................................... 26
7.1 Stopping the recording of a case.......................................................................... 26
7.2 Demo......................................................................................................................... 26
7.3 New patient............................................................................................................... 26
7.4 Maintenance............................................................................................................. 27
7.5 Deleting patient data............................................................................................... 27
7.6 Screen capture......................................................................................................... 27
7.7 Exporting data files.................................................................................................. 27
7.8 Updating events....................................................................................................... 31
7.9 Setting date and time.............................................................................................. 32
7.10 Updating monitor..................................................................................................... 33
7.11 Shutting down .......................................................................................................... 35
8Trouble shooting.......................................................................................................... 35
9Monitor disposal........................................................................................................... 36
10 Environment.................................................................................................................. 36