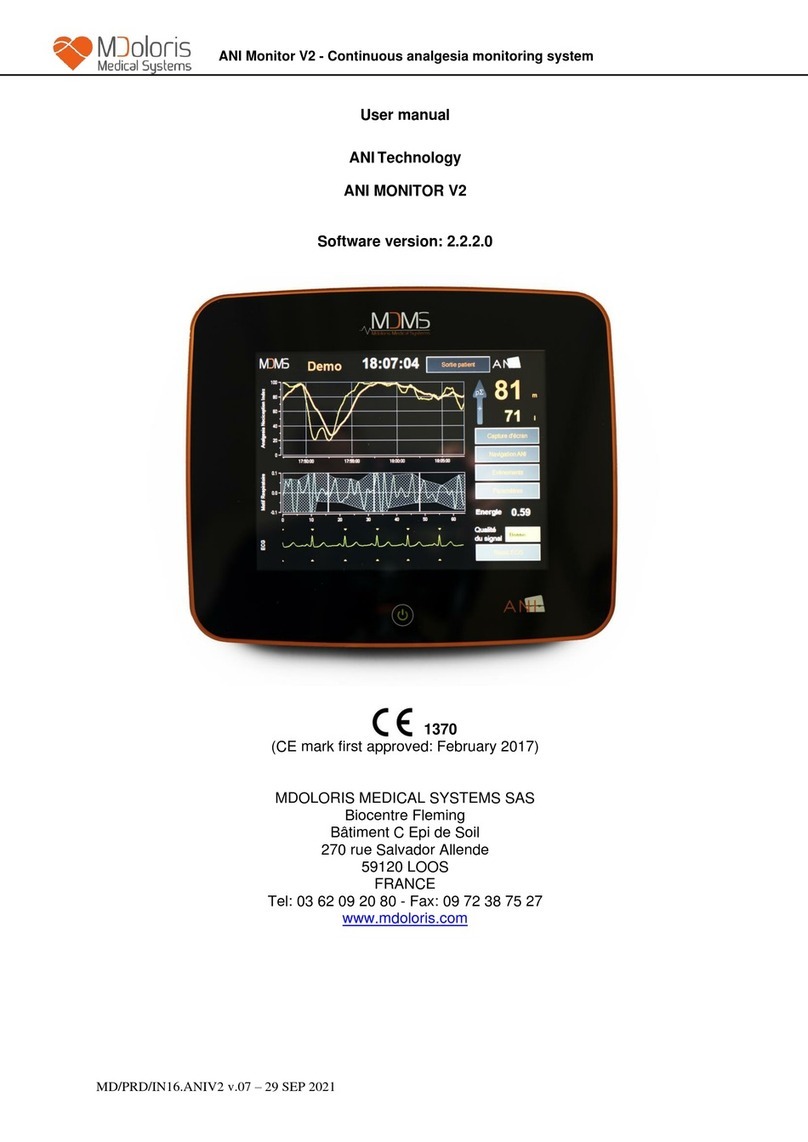
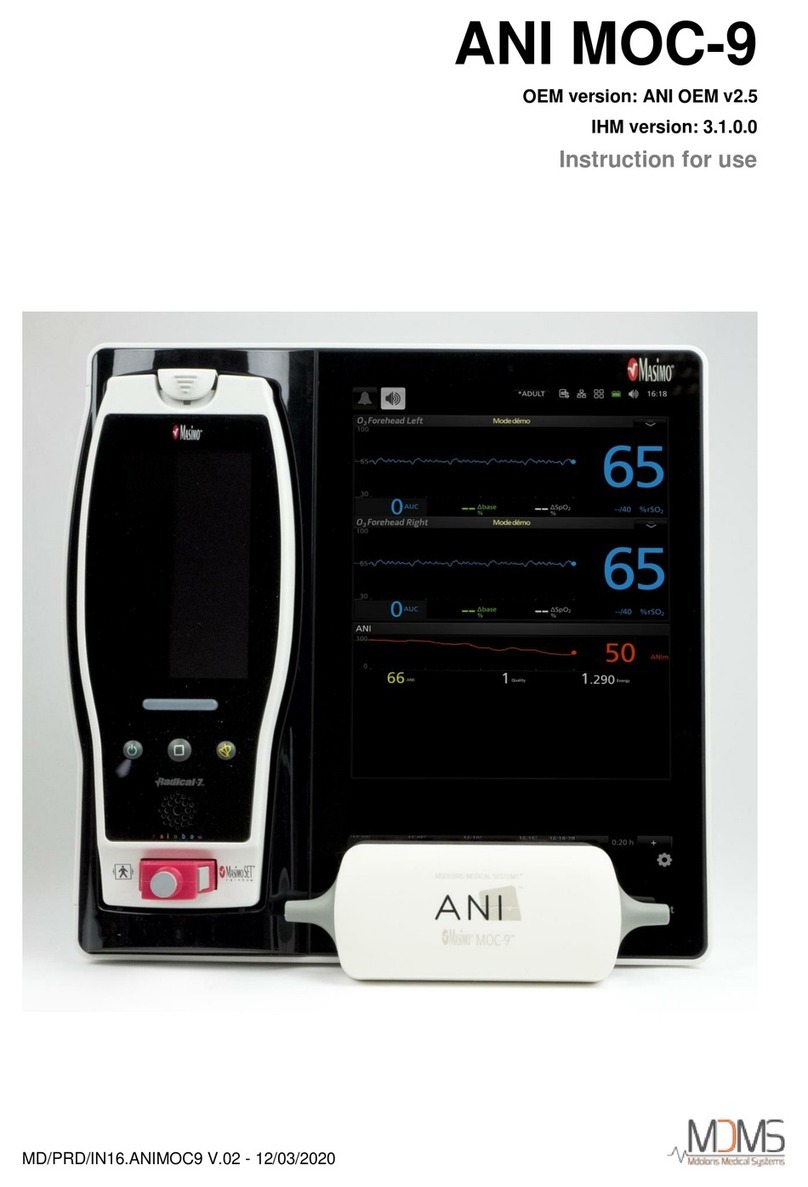
MD/PRD/IN16.ANIMR V.04 - 14/12/2021
Summary
Summary .................................................................................................................. 5
About this manual ................................................................................................... 7
Product Description ................................................................................................ 8
Intended Use ....................................................................................................... 8
Indication for use ................................................................................................. 9
Contraindications ................................................................................................. 9
Side-effects ......................................................................................................... 9
Warning and cautions ........................................................................................... 10
Safety warnings and cautions ........................................................................... 10
Performance warnings and cautions ................................................................. 12
Cleaning and service warnings and cautions .................................................... 13
Compliance warnings and cautions ................................................................... 13
Chapter 1: Technology overview ......................................................................... 15
Chapter 2: System description ............................................................................ 17
BeneVisionTM monitor ........................................................................................ 17
Mindray ANI single slot communication module ................................................ 18
ANI-MR 18
ANI Sensor V1 PLUS ........................................................................................ 19
Chapter 3: Setting Up ANI-MR with ANI Sensors ............................................... 20
Unpacking and inspecting the system ............................................................... 20
Preparation for use ............................................................................................ 20
Connecting ANI Sensor V1 PLUS to the ANI-MR module ................................ 21
Connecting the ANI-MR module ........................................................................ 22
Chapter 4: Operation ............................................................................................ 24
The ANI-MR window ......................................................................................... 24
Mode of operation ............................................................................................. 25
Chapter 5: Troubleshooting ................................................................................. 27