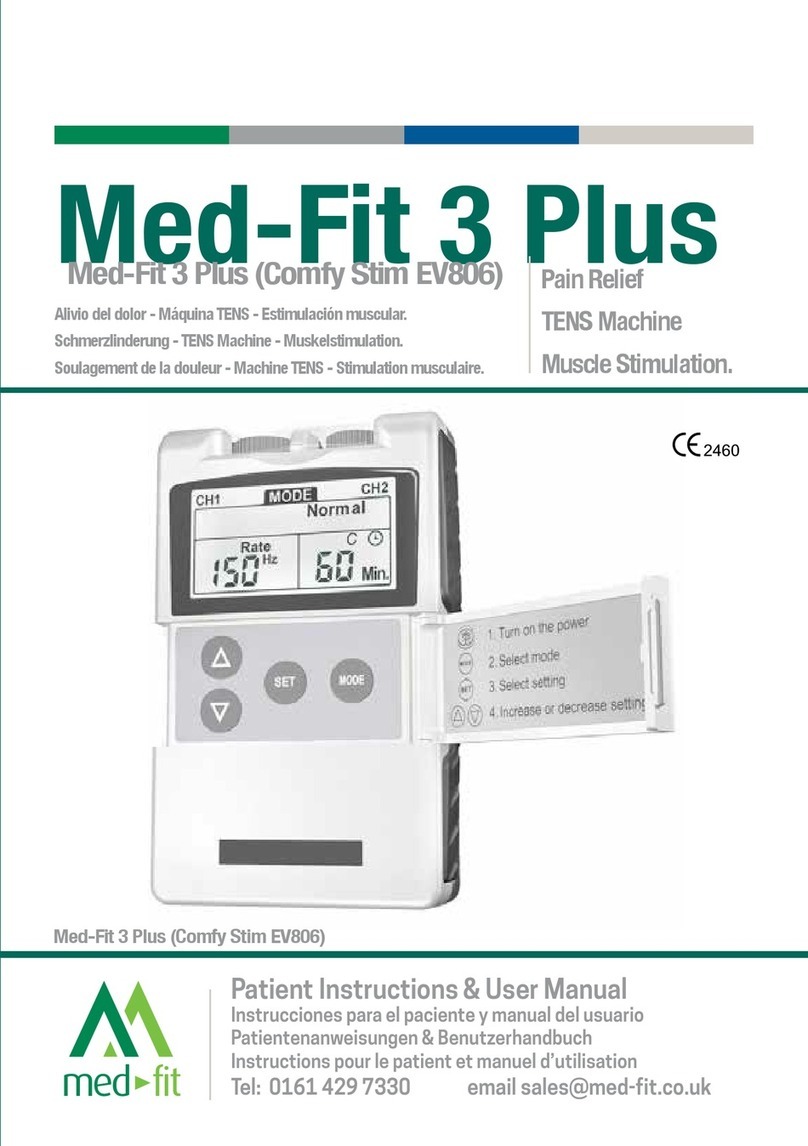
Med-Fit Solo User manual

• Fully Rechargeable
• Very Simple to Operate
• Completely Wireless
Med-Fit Solo
Painless TENS Machine
med fit
Telephone: 0161 429 7330 www.tensmachineuk.com

www.tensmachineuk.com
Med-Fit UK Ltd
Unit 8, Martel Court, S. Park Business Park , Hamilton Road, Stockport, SK1 2AF.
Tel: 0161 429 7330 Fax: 0161 427 0215
Email: [email protected]o.uk www.tensmachineuk.com
Company registration number 08758741 Vat registration number 308286105
med fit
Tec nical Specification:
Channel: x 1
Amplitude: 0~100mA Peak (500 ohm load)
Power Supply: 3.7V/480m A Lithium Polymer Battery
Size: ø60(W) x 19.5(H)mm
Weight: 38.6g (battery included)
Wave form: Symmetrical Rectangular Biphasic Pulse
Output Type: TENS
Treatment Mode: TENS Preset
(2 programmes repeat automatically until the battery runs out.)
P1 -15 minutes (Convential TENS - Frequency: 80Hz / Pulse Width: 70-180µs
P2 -15 minutes (Modulation TENS - Frequency: 80Hz / Pulse Width: 70-180µs
Low Battery Sign: A low battery Logo will flash when the battery is low
2

Telephone: 0161 429 7330
3
Contents
CONTENTS AND GENE AL INFO MATION 4
CONT OLS 4
CHA GING YOU DEVICE 5
FITTING THE ELECT ODES 6
INST UCTIONS FO USE 7
INT ODUCTION 8
FAQS 8
SKIN P EPA ATION 8
ELECT ODES INST UCTIONS 9
WA NINGS & P ECAUTIONS 10
CAUTIONS 11
INT ODUCTION TO TENS 12
WA ANTY 13
EMC INFO MATION 14
G APHIC SYMBOLS 15
www.tensmachineuk.com
4
CONTENTS & GENE AL INFO MATION
CONTENTS & GENE AL INFO MATION
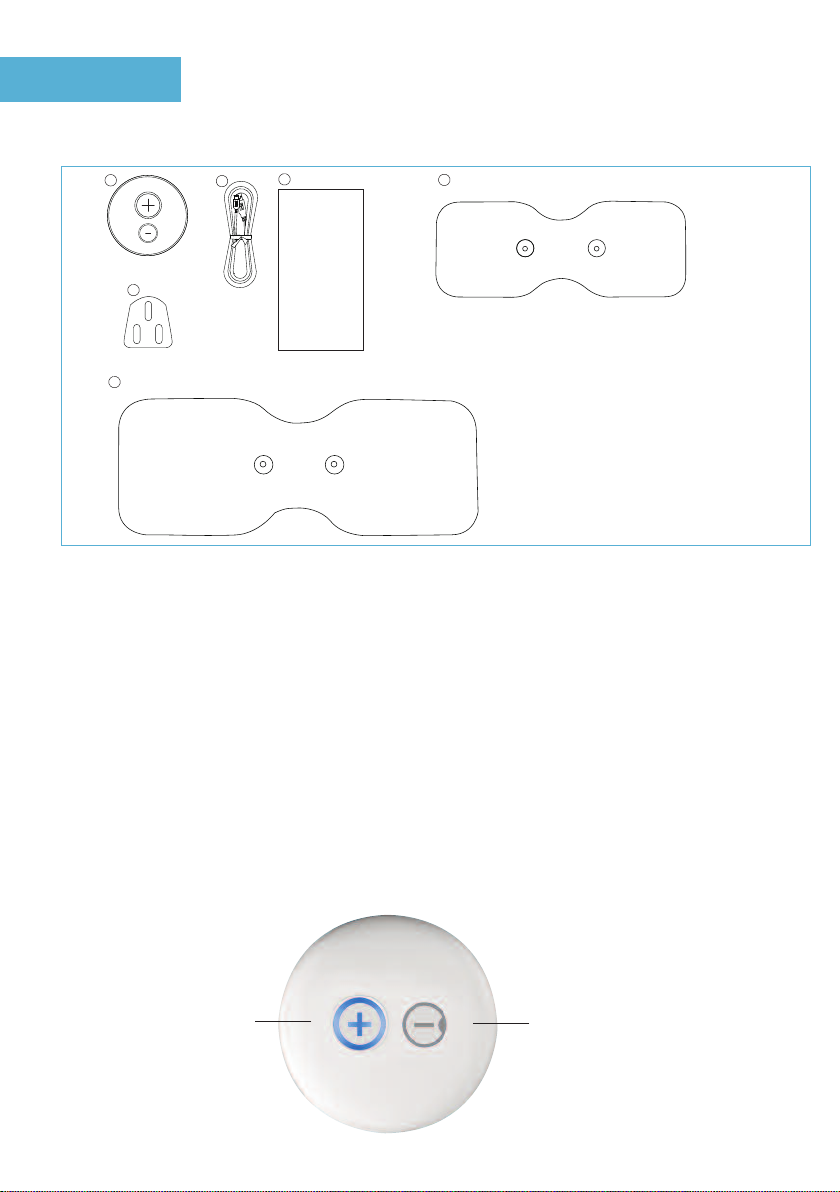
Please check carefully the contents of the
Med-Fit Solo Wireless TENS
1. Wireless TENS Module
2. USB and AC Adaptor Charging Lead
3. AC adaptor
4. nstruction & User Manual
5. Self adhesive Large Electrode 21cm x 8cm
6. Self adhesive Medium Electrodes 14cm x 5.5cm
3
2 4
5
6
1
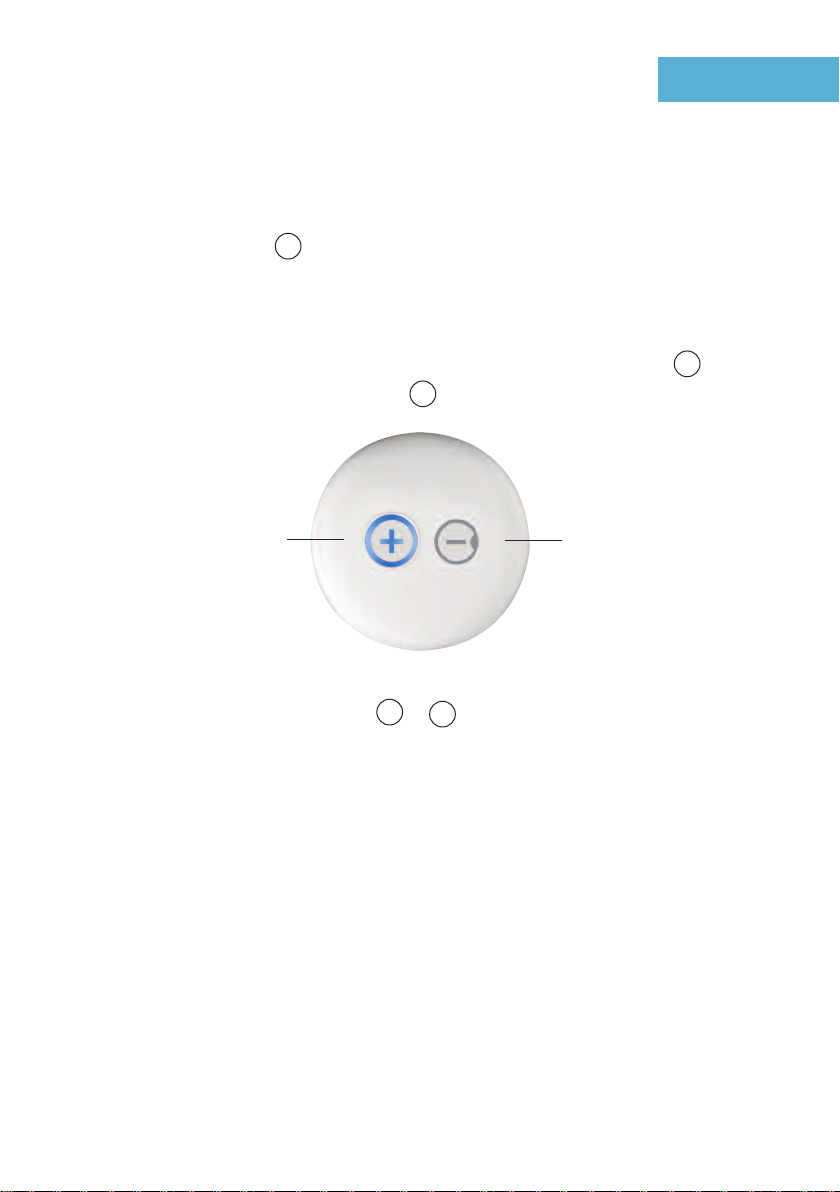
On /Off button and
Intensity up Off buton and intensity down
CONT OLS

Telephone: 0161 429 7330
5
CHA GING YOU DEVICE
To charge your TENS please use the charger and USB cable provided, connect the large end of
the USB cable to the charger and the small end fits into the TENS module as shown in Fig 1.
The USB cable only fits one-way round, please do not force the cable into the USB socket. You
may also charge your TENS from any USB port (typically found on computers).
The button will glow red when charging, once fully charged the button will turn green,
ready for use.
-
A flashing LED indicated your TENS requires charging.
Fig 1.
Fig 2.
Red in charge mode Green fully charged

www.tensmachineuk.com
6
FITTING THE ELECT ODES
There is a choice of two types of which snap connect onto the TENS as shown in Fig 3 and Fig 4.
Fig 3.
Fig 4.
Telephone: 0161 429 7330
7
Each press of the button increases
the intensity.
Blue when switched
Each press of the button
decreases the intensity.
Turning on your TENS
To turn on your TENS hold the button down for 2 seconds the button will now glow blue,
indicating the TENS is turned on.
The TENS device will automatically turn off after 2 minutes when not in use. Prior to turning
off, you will hear a series of beeps indicating that the TENS is switching off. Place the TENS
over the painful area and switch on. You can turn up the intensity by pressing the button.
Each press increases the intensity by 1mA. The button will decrease the
intensity by 1mA with each press see Fig 4.
+
+
-
Recent research has shown TENS to be most effective when used for longer periods typically
11/2- 2 hours at a lower intensity (a level which is just above the sensory level and pleasant in
sensation).
Your TENS, therefore, runs on a continuous program or until it is switched off. A full charge
will typically give 10 hours continuous use.
Please turn off by pressing and holding the or button for 2 seconds before removing
from the treatment area.
-
+
INST UCTIONS FO USE
T EATMENT TIMES AND INTENSITY SETTINGS

www.tensmachineuk.com
8
INT ODUCTION
Thank you for purchasing the Med-Fit Solo Wireless TENS.
t is the most advanced Wireless Stimulator and is manufactured to the highest of medical
standards which fully comply to the Medical Device Directive (M.D.D).
SKIN PATCH TEST
t is recommended that you carry out a patch test before applying your first treatment, To do
this, remove one electrode from the packaging and place on a part of your body which is both
visible and easy to inspect. After 30 minutes, remove the electrode and inspect the area for any
redness or irritations. f no change is noticed, proceed with your first TENS treatment following
the User Guide and nstructions provided. f skin irritation has been noticed, we recommended
the use of sensitive gel electrodes.
FAQS
Question: The sensation is not as strong as when first received my TENS.
Answer: Apply a small amount of water to the gel area as described on page ? of this guide.
Question: need to increase the intensity a little higher each day.
Answer: Applying TENS to the same area each day can dry out your skin. t is important to
wipe the treatment area with warm water before applying your electrodes
SKIN P EPA ATION BEFO E APPLYING YOU
ELECT ODES
t is important that your skin is clean and free from any oils gels or creams before applying
your adhesive pads to the skin.
We recommend however to rub the area to be treated with warm water before applying the
electrodes as this will give the most comfortable stimulation and decrease your skin
resistance.

Telephone: 0161 429 7330
9
ELECT ODE INST UCTIONS
Turn Stimulator OFF before applying or removing electrodes
Application
1. Skin site must be very clean and dry. Dirty, flaky or oily skin will prevent electrodes from adhering to
the skin. f necessary, trim excess hair with scissors. f skin is oily wipe down with an alcohol or
electrode skin prep prior to application. Be sure to wash hands before handling electrodes.
2. Remove electrodes from bag and reseal bag to protect remaining electrodes.
3. Grasping a tiny edge of the electrode, peel and remove electrode from the protective plastic liner. Save
liner for electrode storage.
4. Place electrode onto skin treatment site (as recommended by your clinician) by firmly applying from
the centre of the electrode to the outer edges. Adhesion improves when electrodes reach skin
temperature.
5. f gel appears oversaturated with excessive moisture or perspiration, allow the electrode to air-dry in a
refrigerator with the gel side facing up until the gel regains its tack. f the gel appears dry, try adding a
few drops of water to the gel and allow to rest in a dust-free environment until the gel regains its tack.
Removal and storage
1. Lift a corner of the electrode and slowly peel the electrode off the skin, touching the adhesive gel as
little as possible.
2. Place the electrodes back onto the saved protective plastic liner.
3. While grasping the electrodes connector with one hand, use the other hand to gently twist and
disconnect the lead wire pin from the electrode connector. .
4. Return the electrodes back into the storage bag and reseal tightly to prevent dry-out.
5. Store at room or cool temperature and keep out of direct sunlight.
6. The life of the electrode varies depending on skin conditions, amount of use, storage and climate.
Electrode life may be extended by carefully following the application, removal, and storage instructions.
Caution
1. DO NOT place electrodes on broken skin. f skin irritation develops discontinue use. Consult physician.
Replace electrodes when they do not adhere or when treatment becomes uncomfortable.
2. DO NOT use unit while driving or operating machinery
3. DO NOT wear electrodes when showering, bathing or swimming
4. DO NOT apply electrodes across the head or across the heart or on the front of your neck.
5. Keep electrodes separated during treatment
6. Using stimulation electrodes that are small or incorrectly applied could result in discomfort or skin
burns.

10
www.tensmachineuk.com
WA NINGS & P ECAUTIONS
PLEASE NOTE:
t is imperative that patients read and understand the warnings and precautions before using
this device. Do not allow your machine or electrodes to be used by anyone else, as they are
designed for single patient use only. t is recommended that proper medical advice on the use
of TENS is sought from a Qualified Practitioner (Physiotherapist, Doctor or Nurse) prior to
use, in order to ensure safe and effective treatment. f you are taking any medication please
carry on as normal but seek advice from your Doctor/Healthcare Professional before using
the device.
WARNING! PATIENTS WITH PACEMAKERS MAY NOT BE TREATED WITH TENS
• Do Not use during pregnancy except during labour
(under medical supervision)
• Do Not place electrodes over the Carotid Sinus
• Do Not use on broken or damaged skin
• Do Not place electrodes close to the eyes or in the mouth.
• Do Not use TENS whilst driving or operating machinery.
TENS is unsuitable and s ould not be used in t e following situations.
• Persons suffering from conditions where the circulation is impaired.
• Epilepsy, Heart Condition or any form of Malignancy.
• Patients with poor skin sensation and non-compliant patients who are emotionally
disturbed or have dementia.
• Over metal implants or in conjunction with sleep apnea or heart monitors.
You should be aware that TENS units provide symptomatic relief only and are not considered
curative.

11
Telephone: 0161 429 7330
WA NINGS
1. The long term effects of chronic electrical stimulation are unknown.
2. Stimulation should not be applied over the carotid sinus nerves, particularly in patients with a
known sensitivity to the carotid sinus reflex.
3. Stimulation should not be applied over the neck or mouth. Severe spasm of the laryngeal and
pharyngeal muscles may occur and the contractions may be strong enough to close the airway or
cause difficulty in breathing.
4. Stimulation should not be applied transthoracically in that the introduction of electrical current into
the heart may cause cardiac arrhythmias.
5. Stimulation should not be applied transcerebrally
6. Stimulation should not be applied over swollen, infected, inflamed areas or skin eruptions, eg,
phlebitis, thrombophlebitis, varicose veins etc.
7. Stimulation should not be applied over or in proximity to cancerous lesions.
Contraindication
Electrical stimulators should not be used on patients with cardiac demand pacemakers.
Adverse Reactions
On rare occasions skin irritation and burns beneath the electrodes have been reported with the use of
electrical stimulators. f irritation occurs, discontinue use and consult your Healthcare Professional.
CAUTIONS
1. Safety of powered muscle stimulators for use during pregnancy has not been established.
2. Caution should be used for patients with suspected or diagnosed heart problems.
3. Caution should be used in the presence of the following:
a. When there is a tendency to haemorrhage following acute trauma or fracture;
b. Following recent surgical procedures when muscle contraction may disrupt the healing process;
c. Over the menstruating or pregnant uterus; and
d. Over areas of the skin which lack normal sensation.
4. Some patients may experience skin irritation or hypersensitivity due to electrical stimulation or
electrical conductive medium. Using an alternate conductive medium, or alternate electrode
placement can usually reduce the irritation.
5. Electrode placement and stimulation settings should be based on the guidance of the prescribing
practitioner.
6. Powered muscle stimulators should be kept out of the reach of children.
7. Powered muscle stimulators should be used only with the leads and electrodes recommended for
use by the manufacturer.
8. Portable powered muscle stimulators should not be used while driving, operating machinery or
during any activity in which involuntary muscle contractions may put the user at undue risk of
injury.

12
www.tensmachineuk.com
INT ODUCTION TO TENS
W at is TENS?
Transcutaneous electrical nerve stimulation is a pain control treatment. t is often called TENS
for short.
A TENS unit is a portable, pocket-sized, battery-powered device.
The TENS unit uses mild, safe electrical signals to help control pain and delivers the electrical
signal to the body through self-adhesive conductive electrodes.
How does TENS work?
The most common TENS programmes use
high-frequency stimulation, which is the first choice for both acute and chronic pain.
High-frequency stimulation sends impulses to the nervous system’s own pain-inhibiting
mechanisms, which block the pain.You can use it as often and as long as you like, but each
treatment should last at least 1 hour.
Another type of TENS is low-frequency stimulation. Low-frequency TENS treatment can
alleviate pain by stimulating muscles to release the body’s own morphine-like substances,
called endorphins.
During t e TENS treatment
f your muscles start to twitch, this may mean that the TENS signals are too strong or too fast.
f you cannot feel any tingling at all, this may mean that the signal is too weak or too slow.
The electrodes should be removed at least once a day if the TENS treatment is used around the
clock. The skin under the electrodes must be checked to see if it is red or tender. The skin
should also be cleaned and dried while the electrodes are off. Apply lotion to your skin where
the electrodes were placed. The electrodes should be applied to a different area for each new
treatment. This will help prevent the skin from becoming red or sore.
TENS can be used for
TENS can be used to treat most types of pain where the cause has been determined including:
• Arthritis
• Back Pain Post Herpetic
• Bruising Neuralgia
• Calf Strain
• Dead Leg
• Fibrositis Finger Pain
• Rheumatism
• Sciatica
• Headaches
• Migraines
• Shoulder Pain
• Sleeplessness
• Knee Pain
• Lumbago Muscle
• Stress
• Sports njuries
• Tennis Elbow
• Neck Pain
• Neuralgia
• Osteoarthritis

13
Telephone: 0161 429 7330
HOW HIGH SHOULD I TU N THE INTENSITY?
Everybody reacts differently to TENS Stimulation so it is important that you increase the
intensity (sensation feeling) to the correct level.
ncrease the intensity to a sensation which is comfortable and always perceptible; never turn
up to a level which is strong and uncomfortable.
You may use TENS if required for long periods of time to combat long term chronic pain;
however, please remember to place the electrodes in slightly different areas around the
painful site, as this will help reduce skin irritation.
The most up to date research in TENS treatment times indicates that a minimum of 1 hour to
11/2hours is required for effective pain relief. Your TENS may be used for much longer periods
and you may find treatment times of 3 to 4 hours may work best for you.
Please remember that the intensity level is always kept at a pleasant sensation, never increase
the intensity to uncomfortable levels as this can possibly have a detrimental effect on your
results.
HOW LONG SHOULD A TYPICAL T EATMENT
TIME LAST
LIMITED WA ANTY
Med-Fit warrants to the initial Purchaser (“Purchaser”) (and to no other person) that the
product with the exclusion of accessories such as chargers, rechargeable batteries,
electrodes, lead wires, self-adhesive electrodes and the component parts thereof, distributed
or manufactured for one year from the initial date of purchase from Med-Fit (“the Warranty
Period”).
Accessories including, but not limited to chargers, rechargeable batteries, electrodes, lead
wires and adhesive electrodes are excluded from the warranty and sold ”AS S’ because their
structure is such that they may be easily damaged before or during use.
Limited of Liabilities and Disclaimer of Warranties
Med-Fit sole obligation in the case of any breach of its warranties set forth in the paragraph
above, shall be, at Med-Fit option, to repair or replace the Product without charge to Purchaser
or to refund the purchase price of the Product. n order to recover under this Warranty,
Purchaser must send Med-Fit written notice of the defect (setting forth the problem in
reasonable detail) prior to expiration of the Warranty Period, and within 30 days of discovery of
the defect.

www.tensmachineuk.com
14
Guidance and manufacturer s declaration - electromagnetic emissions
The device is intended for use in the electromagnetic environment
specified below. The customer or the user of the device should
assure that it is used in such an environment.
Emissions test
Compliance
Electromagnetic environment -
guidance
RF emissions Group 1 The device must emit electromag-
netic energy in order to perform its
CISPR 11 intended function. Nearby electronic
equipment may be affected.
RF emissions Class B The device is suitable for use in all
CISPR 11 establishments other than domestic
Harmonic emissions Class C those directly connected to the public
IEC 61000-3-2 low-voltage power supply network that
Voltage fluctuations Complies supplies buildings used for domestic
/ flicker emissions purposes.
IEC 61000-3-3
¤”› ד⁄—Œ”flÃ×—“
The device complies with current EMC regulations.
The radio frequency emissions of the device are extremely low and in
all probability do not cause any interference with other devices in the
proximity.It is recommended that you do not place the device on top of
or close to other electronic devices.
Guidance and manufacturer s declaration - electromagnetic immunity
The device is intended for use in the electromagnetic environment specified
below. The customer or the user of the device should assure that it is used
in such an environment.
IMMUNITY test
Electrostatic
discharge (ESD)
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips, short
interruptions and
voltage variations on
power supply
input lines IEC 61000-
4-11
Power frequency
(50/60 Hz) magnetic
field IEC 61000-4-8
IEC 60601 test
level
!6 kV contact
!8 kV air
!2 kV for power
supply lines
!1 kV line(s) to
line(s) and neutral
<5 % UT
(>95 % dip in UT)
for 0,5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5 s
3 A/m
Compliance level
!6 kV contact
!8 kV air
!2 kV for power
supply lines
!1 kV line(s) to
line(s) and neutral
<5 % UT
(>95 % dip in UT)
for 0,5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5 s
Not applicable
Electromagnetic environment -
guidance
Floors should be wood, concrete or
ceramic tile. If floors are covered
with synthetic material, the relative
humidity should be at least 30 % .
Mains power quality should be that
of a typical commercial or hospital
environment.
Mains power quality should be that
of a typical commercial or hospital
environment.
Mains power quality should be that
of a typical commercial or hospital
environment. If the user of the
device requires continued
operation during power mains
interruptions, it is recommended that
the device be powered from an
uninterruptible power supply or a
battery.
Not applicable
NOTE UTis the a.c. mains voltage prior to application of the test level.

Telephone: 0161 429 7330
15
Separation distance according to frequency of transmitter
m
150KHz bis 800MHz
Rated maximum
output power
of transmitter
W
80MHz bis 2.5GHz
0,01 0,12
0,1 0,38
1 1,2
10 3,8
100 12
80MHz bis 800MHz
0,12 0,23
0,38 0,73
1,2 2.3
3.8 7,3
12 23
For transmitters rated at a maximum output power not listed above,
the recommended separation distance in metres (m) can be estimat-
ed using the equation applicable to the frequency of the transmitter,
where P is the maximum output power rating of the transmitter in
watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the high-
er frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromag-
netic propagation is affected by absorption and reflection from struc-
tures, objects and people.
Recommended separation distances between
portable and mobile RF communications equipment and the device
The device is intended for use in an electromagnetic environment in
which radiated RF disturbances are controlled. The customer or the user of the
device can help prevent electromagnetic interference by maintaining a
minimum distance between portable and mobile RF communications equipment
(transmitters) and the device as recommended below, according to the
maximum output power of the communications equipment.
d= 1,2 d= 1,2 d= 2,3
¤”› ד⁄—Œ”flÃ×—“
2460

2460
med fit
Telephone: 0161 429 7330 www.tensmachineuk.com
Table of contents
Other Med-Fit Medical Equipment manuals