– 1 –
1. SAFETY PRECAUTIONS
The following precautions should always be exercised with the use of all electro-medical equipment to ensure safety to
all involved parties - user(s), patient(s), etc. Please read carefully and follow this owner’s manual.
1-1. TRAINING
1. This equipment should only be used under the supervision of a trained physician in a medical facility. Do NOT use
in other locations or for any purposes other than the intended application.
1-2. INSTALLATION
1. This equipment should NEVER be installed or used in areas where the unit could get wet or be exposed to any
environmental conditions such as high temperature, humidity, direct sunlight, dust, salt, etc., which could
adversely affect the equipment.
2. This equipment should NEVER be installed or used in the presence of flammable or explosive gases or
chemicals.
3. This equipment should NEVER be installed, used or transported in an inclined position nor should it be subjected
to impact or vibration.
4. For safety reasons, this equipment must be properly grounded. This equipment should be connected to a three (3)
prong hospital grade receptacle in U.S.A. or Canada.
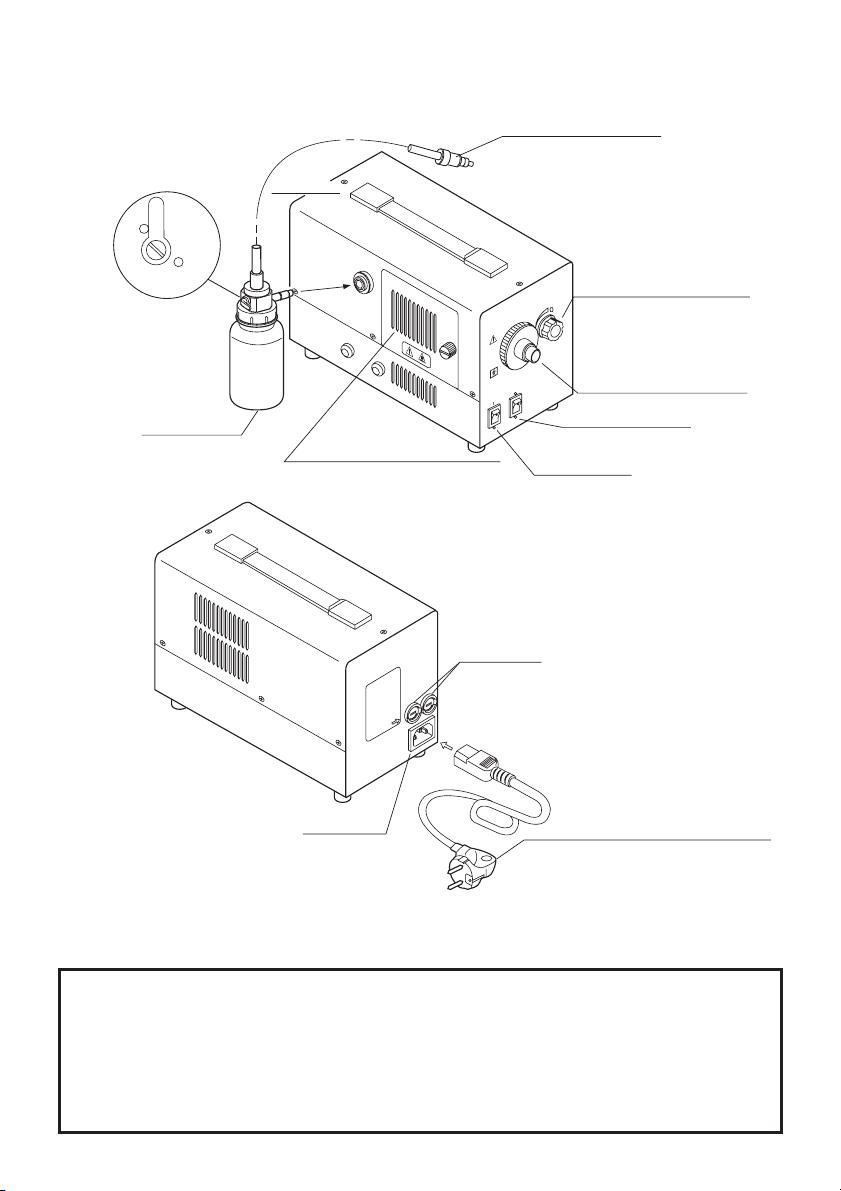
5. Ensure that all power requirements are met and conform to those specified on the rating plate located on the rear
panel.
6. Do NOT block the air intake vent of this equipment.
7. Do NOT allow the power cord to become twisted, crushed or pulled taut.
8. When using an isolation transformer for any ancillary equipment, ensure the power requirements of the devices
do not exceed the capacity of the isolation transformer. For further information, contact your local PENTAX
distributor.
1-3. PRIOR TO USE
1. Confirm that this equipment functions properly and check the operation of all switches, indicators, etc.
2. To prevent electrical shock when used with endoscopes, this equipment is insulated type BF electro-medical
equipment.
Do NOT allow it to be grounded to other electrical devices being used on the patient. Rubber gloves should always
be worn to prevent grounding through user(s).
3. Confirm that other devices used in conjunction with this equipment function properly and that these other devices
will not adversely affect the operation or safety of this equipment. If any component of the endoscopic system is
not properly functioning, the procedure should not be performed.
4. Check and confirm that all cords or cables are connected correctly and securely.
1-4. DURING USE
1. To prevent electric shock, the endoscope and/or any other ancillary device should NEVER be applied directly to
the heart.
2. Make sure that no contact is made between the patient and this equipment.
3. The light emitted by the halogen lamp is extremely intense. Avoid looking directly at the light exiting the
endoscope and/or this equipment.
4. To protect the users eyes and avoid risk of thermal injury during an endoscopic examination, use only the
minimum amount of brightness required.
5. During clinical procedures, avoid unnecessary prolonged use which could compromise patient/user safety.
6. Continually monitor this equipment and the patient for any signs of irregularities.
7. In the event that some type of irregularity is noted to the patient or this equipment, take the appropriate action to
ensure patient safety.
8. If the operation of any of the components of the endoscopic system fails during the procedure and the visualization
of the procedure is lost or compromised, place the endoscope in the neutral position and slowly withdraw the
endoscope.
9. This equipment should only be used according to the instruction and operating conditions described in this manual.
Failure to do so could result in compromised safety, equipment malfunction or instrument damage.