mesoskinline MESO POWER DEVICE MS-3 User manual

MESO POWER DEVICE MS-3
OPERATING MANUAL
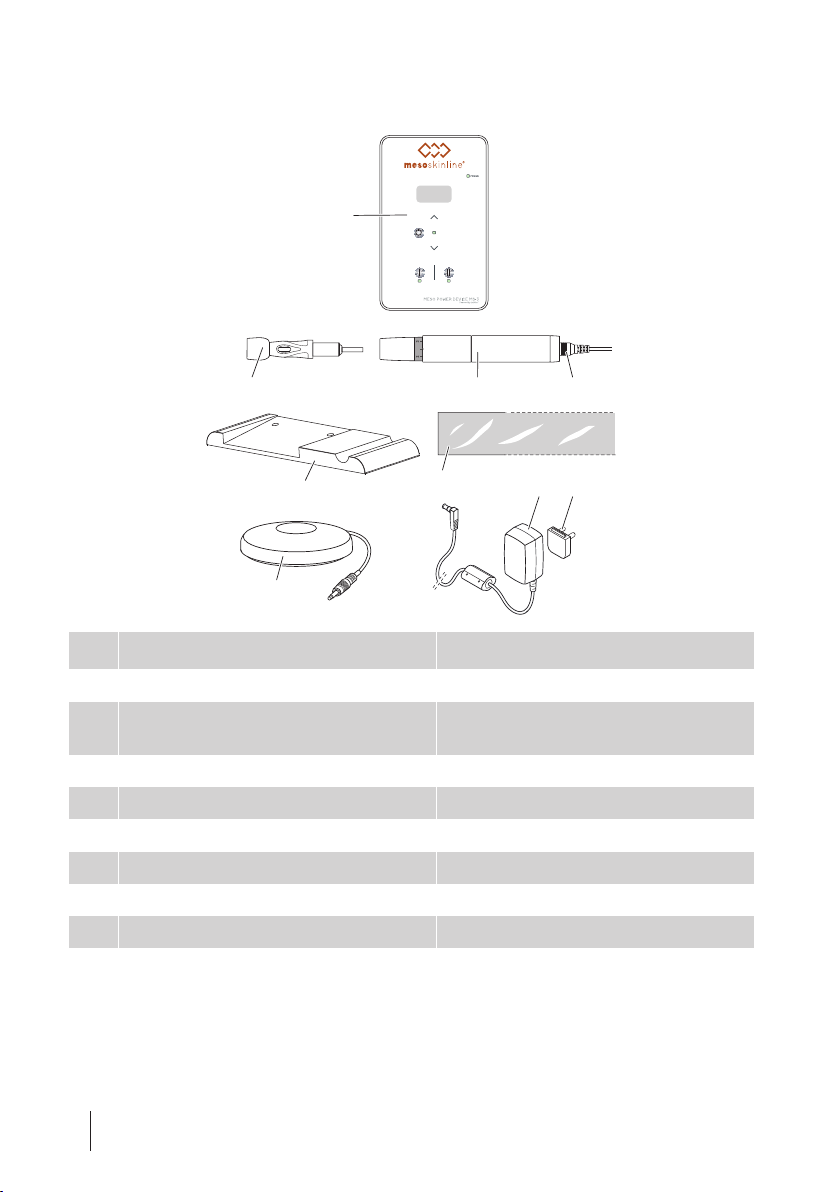
PRODUCT OVERVIEW
6
5
1
47 8
2* 3a 3b
ARTICLE DESIGNATION ARTICLE DESCRIPTION
1 Controller MAD12901
2* Needle cartridges "4.3Accessories and spare parts" on
page16
3a Handpiece CMN50
3b Handpiece cable 5E-G765
4 Handpiece tray FG-AD1.0-1
5* Handpiece cover E-0610
6 Foot switch E-1010
7 Power supply E1165
8 Country-specific adapter EU: E-1154
* Not included with the delivery
2 EN MESO POWER DEVICE MS-3

6 7 8 9
1
2
3
4
5
BUTTONS AND CONNECTIONS
1 Display
2Treatment ON/OFF button (white LED)
3Select handpiece I/II buttons (white LED)
4Power LED indicator ( ) (white LED)
5Increase frequency ( ) button / decrease frequency ( ) button
6 Connection for foot switch
7 Connection for handpiece I and II
8 Connection for power supply
9Device ON/OFF button
3ENMESO POWER DEVICE MS-3

4 EN MESO POWER DEVICE MS-3
English
Version 1.0 – 01/2019
Translation of the original operating manual. The original operating manual was generated in German.
CONTENTS
1 About this manual........................................................................................................................6
1.1 Presentation of warning symbols................................................................................................................6
2 Important safety instructions ...................................................................................................7
2.1 General safety instructions ..............................................................................................................................7
2.2 Product-specific safety instructions .........................................................................................................8
2.3 Important health and safety precautions ..............................................................................................8
2.4 Contraindications and side effects............................................................................................................ 10
2.5 Required qualification (user requirements)......................................................................................... 11
2.6 Intended purpose, scope of application and proper use.......................................................... 12
2.7 Symbols on the product......................................................................................................................................13
3 Scope of delivery .........................................................................................................................15
4 Product information....................................................................................................................16
4.1 Technical data.............................................................................................................................................................16
4.2 Operating conditions .............................................................................................................................................16
4.3 Accessories and spare parts...........................................................................................................................16
5 Getting started..............................................................................................................................17
5.1 Positioning the device..........................................................................................................................................18
5.2 Connecting the power supply ........................................................................................................................ 19
5.3 Connecting the foot switch (optional).....................................................................................................20
5.4 Connecting the handpiece ............................................................................................................................... 21
5.5 Cleaning and disinfecting the equipment............................................................................................. 21
5.6 Inserting or replacing the needle cartridge.........................................................................................22
5.7 Pulling the handpiece cover on .................................................................................................................... 23
5.8 Checking equipment.............................................................................................................................................. 24
6 Use ...................................................................................................................................................24
6.1 Switching the control unit to operating mode or standby mode........................................ 24
6.2 Setting the needle protrusion depth......................................................................................................... 25
6.3 Setting the penetration frequency..............................................................................................................25
6.4 Switching the handpiece on and off.........................................................................................................26

5ENMESO POWER DEVICE MS-3
7 Cleaning and maintenance .......................................................................................................27
7.1 Inspection .......................................................................................................................................................................27
7.2 Material compatibilities........................................................................................................................................27
7.3 Disinfecting surfaces ............................................................................................................................................28
7.4 Cleaning surfaces....................................................................................................................................................28
8 Transport and storage conditions...........................................................................................29
8.1 Controller, handpiece with handpiece cable, power supply and foot switch........... 29
8.2 Needle cartridge .......................................................................................................................................................29
9 Disposal..........................................................................................................................................30
10 Questions and problems............................................................................................................30
11 Guarantee ......................................................................................................................................31
12 Declaration of conformity .........................................................................................................32

6 EN MESO POWER DEVICE MS-3
1 ABOUT THIS MANUAL
This operating manual applies to the MESO POWER DEVICE MS-3 (AD12901) and its ac-
cessories. It contains important information about setting up, operating and looking after the
device safely and as intended for use.
This operating manual is restricted to all information essential for the safe use of the device.
For further information, required for the safe use of the device and its accessories, please
also read the following additional documentation:
OSafety datasheets for disinfectants and cleaning materials
OSafety at the workplace requirements and statutory provisions for microneedling
1.1 Presentation of warning symbols
Warning symbols highlight the danger to persons or objects and are set up as follows:
SIGNAL WORD
Type of danger
Consequences
EPrevention
ELEMENT MEANING
Indicates risk of injury
Signal word Indicates the seriousness of the hazard (see following table)
Type of hazard the type and source of the hazard
Consequences describes possible consequences if ignored
Prevention indicates how the hazard can be prevented
SIGNAL WORD MEANING
Danger Indicates a danger which will certainly result in severe or fatal injuries if the
danger is not avoided
Warning Indicates a danger which could result in severe or fatal injuries if the
danger is not avoided

7ENMESO POWER DEVICE MS-3
SIGNAL WORD MEANING
Caution Indicates a danger which may cause slight to medium-severe injuries if the
danger is not avoided
Attention Indicates a potential risk of damage to the environment, property or the
equipment if the danger is not avoided
Symbols in this operating manual
SYMBOL MEANING
EAction required
OBullet points
- Sub-bullet points
2 IMPORTANT SAFETY INSTRUCTIONS
2.1 General safety instructions
ERead this operating manual carefully and in full.
EKeep this operating manual in a location which is accessible to all those using, cleaning,
disinfecting, storing or transporting the device.
EAlways include this operating manual when transferring ownership of the device.
EFollow the safety regulations for the microneedling in your country. Keep your workplace
hygienically clean and ensure that you have adequate lighting.
EOnly use the device, its accessories and all handpiece cables when in perfect condition.
EUse only original needle cartridges, accessories and spare parts from
MT.DERM GmbH.

8 EN MESO POWER DEVICE MS-3
2.2 Product-specific safety instructions
ENever modify the device, the needle cartridges or other accessories.
EChildren must be supervised and prevented from playing with the device.
EPrevent fluids from getting inside the device, the handpiece, the foot switch or the power
supply.
EAvoid sources of interference by ensuring that portable or mobile radio devices are not
operated in the vicinity of the device.
EWhen being handled, protect the handpiece and the handpiece cable from contamination
by bodily fluids or substances contaminated with bodily fluids by means of a handpiece
cover (see chapter"5.6Inserting or replacing the needle cartridge" on page22).
EIf the device is not being used, disconnect it from the power supply and place the hand-
piece in the handpiece tray so that it cannot roll away or fall.
EObserve the technical data provided in this operating manual and comply with the oper-
ating, transport and storage conditions (see chapter "8Transport and storage condi-
tions" on page29).
ESend the device to a specialist retailer for inspection if there are signs of damage, if it
does not function as normal or if liquids enter the device or handpiece.
EIt is recommended that the device is sent to a specialist retailer for inspection at regular
24 month intervals. In doing so, refer to chapter"7.1Inspection" on page27.
2.3 Important health and safety precautions
Please observe the following instructions in order to prevent contaminations or infectious
diseases being transmitted to the customers or users during treatment:
EFollow all of the steps for disinfecting the equipment before treatment (see chapter
"5.5Cleaning and disinfecting the equipment" on page21).
EUse disposable gloves made from nitrile or latex during the treatment and disinfect these
before use. Observe the applicable directives for your country when selecting suitable
disinfectants.
EClean the respective area of the customer's skin with a mild cleaning agent and disin-
fectant before treatment. Observe the applicable directives for your country when select-
ing suitable disinfectants.
EIf desired for the treatment, use only topical and sterile accompanying preparations that
have been tested and approved for insertion into human skin. Observe the applicable dir-
ectives for your country when selecting these. If substances that are not intended for
cosmetic microneedle treatment or whose sterility is not guaranteed, are introduced into
the skin, this can result in infections or possible side effects. Observe the accompanying
information for the substances used.

9ENMESO POWER DEVICE MS-3
EUse only new, sterile packaged needle cartridges for each customer. Before treatment
ensure that the packaging is undamaged and the expiry date has not passed. Note the
batch number of the needle cartridge used in the corresponding customer file in order to
be able to pass this on to the manufacturer in the event of any problems.
Eneedle cartridges are sterile disposable products (consumables) and are only ever per-
mitted to be used once.
EDispose of used or faulty needle cartridges as well as needle cartridges whose pack-
aging is damaged in a non-penetrable sharps container in accordance with the regula-
tions applicable in your country.
EBefore the treatment, switch the handpiece off and check that the needles have been
completely withdrawn into the needle cartridge. If this is not the case, dispose of the
needle cartridge immediately.
EEnsure that needle cartridges never come into contact with contaminated items, such as
clothing for example. Contaminated needle cartridges must not be used but rather dis-
posed of immediately.
EDuring use, the handpiece, the handpiece cable and the device including the integrated
handpiece tray must be completely covered with protective film. The handpiece must be
covered with the protective film sleeve before attaching the needle cartridge (see
chapter "5.7Pulling the handpiece cover on" on page23).
ECheck at regular intervals whether the handpiece is visibly contaminated by bodily fluids
running back or by the pigment ink being used. In this case, observe the contents of
chapters "7.4Cleaning surfaces" on page28 and "7.2Material compatibilities" on
page27, as well as the chapter"2.2Product-specific safety instructions" on page8.
EIf a local anaesthetic is being used, this should be thoroughly removed before the treat-
ment.
EAlways hold the handpiece firmly before switching it on or place it in the handpiece tray.
If an unsecured handpiece is switched on, it may move uncontrollably through vibrations
and prick or cause injury (see chapter "6.4Switching the handpiece on and off" on
page26).
Injuries through contaminated needles or needle cartridges can result in the transmission of
diseases (see chapter "5.6Inserting or replacing the needle cartridge" on page22). In the
event of being injured by a contaminated needle, seek immediate medical attention from a
physician!
In order to prevent the intensity of the treatment exceeding the extent desired:
EAvoid risk of injury due to the needles protruding too deeply (see chapter "6.2Setting
the needle protrusion depth" on page25).
EAvoid risk of injury due to the penetration frequency being too high (see chapter
"6.3Setting the penetration frequency" on page25).

10 EN MESO POWER DEVICE MS-3
Please be sure to inform your customer to avoid contact with recently treated skin. Recently
treated areas of skin should also be protected from
OContamination and
OUV and solar radiation.
Additional irritants such as
OVisits to swimming pools or saunas
OAbrasive or chemical peelings
OHair removal at the treated areas or
OSelf-tanning products
should be avoided by the customer in the first two to three days after the treatment.
2.4 Contraindications and side effects
The following contraindications and side effects are the results of a thorough analysis of
the professional clinical literature concerning microneedle treatment. If the person respons-
ible for the treatment should have the smallest doubt that the safety of the customer being
treated cannot be guaranteed, for example due to secondary illnesses or conditions, the
treatment should be withheld or stopped immediately.
2.4.1 Contraindications
With the following contraindications, no microneedling treatment should be undertaken:
OHaemophilia or other blood-clotting disorders
OCurrently taking blood thinning medication (e.g. Warfarin, Heparin, aspirin, acetylsalicylic
acid)
OUncontrolled diabetes mellitus
OAny form of active acne in the area of the treatment
ODermatosis (e.g. Skin tumours, keloids or extreme tendency for keloid formation, solar
keratosis, warts and/or moles) in the area of the treatment
OOpen wounds and/or eczema and/or rashes in the area of the treatment
OScars in the treatment area
OSystemic infections and infectious diseases (e.g. Hepatitis type A,B,C,D,E orF; HIV in-
fection) or acute local skin infections (e.g. herpes, rosacea)
ODuring chemotherapy, radiotherapy or high-dosage corticosteroid therapy (recommenda-
tion: from four weeks before the start until four weeks after the end of the therapy)
OUp to twelve months after a plastic surgery operation in the area to be treated
OUp to six months after filler injections in the area to be treated
OAllergy to topical anaesthetics (local anaesthetic)
OUnder the influence of alcohol and/or drugs
OPregnancy and lactation

11ENMESO POWER DEVICE MS-3
The treatment of mucous membrane and eyeballs is strictly forbidden.
The treatment must be immediately aborted in the event of:
OExcessive perception of pain
OFainting/dizziness
2.4.2 Side effects
Often:
OLocalised bleeding in the area of the treated skin
OPain and discomfort on the first day after the treatment
OShort-term inflammatory reactions, erythema and/or oedema up to six days after the
treatment
OSkin irritations (e.g. itching or temperature increase), which normally die out over the first
12to 72hours after the treatment
OFormation of scabs, which normally recede in the first five days
OTemporary peeling of the skin which normally subsides within eight days
Seldom:
OFormation of herpes simplex virus type I (HSV-I) blisters
OFormation of small pustules or milia as a result of inadequate cleaning of the skin prior to
treatment
OHyperpigmentation with the body’s own pigments, in particular with darker skin types, but
completely cleared up within a few weeks
OInflammatory reactions, haematoma, erythema and oedema
ORetinoid reaction (from slight reddening to peeling of the skin)
As a matter of principle, recently treated skin areas should be protected from UV and solar
radiation.
2.5 Required qualification (user requirements)
The device and its accessories may not be used by persons with impaired physical, sensory
or mental capabilities or by children. The device and its accessories may not be used by per-
sons with no experience or knowledge unless they are being supervised or instructed (train-
ing).
The device may only be used by persons trained in the following:
OQualification for cosmetic microneedling
OBasic knowledge of microneedling treatment (see chapter "6.3Setting the penetration
frequency" on page25 and "6.4Switching the handpiece on and off" on page26)

12 EN MESO POWER DEVICE MS-3
OKnowledge of hygiene and safety regulations (see chapter "2.3Important health and
safety precautions" on page8)
OKnowledge of risks and side effects (see chapter"2.4.1Contraindications" on page10
and "2.4.2Side effects" on page11)
2.6 Intended purpose, scope of application and proper use
2.6.1 Intended purpose
The device was developed for the cosmetic microneedling (meta therapy) of healthy skin for
the purpose of skin rejuvenation. In doing so, the self-regeneration of the skin is stimulated
by creating very small punctures in the epidermis (upper skin layer).
With cosmetic microneedling, the epidermis is punctured with minimal invasiveness without
the skin incurring open wounds. Afterwards, the skin requires only a very short regeneration
phase in which the epithelial function will be restored.
2.6.2 Scope of application
Applications for cosmetic microneedling for increased epidermal penetration are:
OTreatment of lines/wrinkles for skin rejuvenation
OStimulation of skin cell activity via high-frequency and non-invasive perforation of the
epidermis
OImprovement of the blood flow in the skin
2.6.3 Proper intended use
The treatment must be carried out in a dry, clean and smoke-free environment as well as
under hygienic conditions. The device must be prepared, used and looked after as de-
scribed in this operating manual. In particular, the applicable requirements concerning work-
place design must be observed and the materials to be used must be sterile.
Intended use also includes the assumption that this operating manual and in particular
chapter "2Important safety instructions" on page7, have been read in full and understood.
Intended use also assumes the following consumer groups exclusively:
OAdult men and women who are in good health.
Improper use is when the device or its accessories are used in a manner other than as de-
scribed in this operating manual or if the operating conditions have not been complied with.
The treatment of contraindications in particular is forbidden (see chapter "2.4.1Contraindic-
ations" on page10).
13ENMESO POWER DEVICE MS-3
2.7 Symbols on the product
The symbols described below can be found in this operating manual, on the device and on
its accessories or the packaging:
SYMBOL MEANING
Device complies with the requirements of directive 2006/42/EC & 2004/108/EC
Attention!
Housing offers protection from coarse dust and water drops
Housing offers protection from jets of water
Housing offers protection from foreign objects thicker than 1mm
Applied part, type B: Applied part provides safety from electrical shock and leakage
currents
Handpiece
Foot switch
DC connection, inner pin positive
DC
AC
Observe operating manual!
Manufacturer
Date of manufacture
Catalogue number, order number
Serial number

14 EN MESO POWER DEVICE MS-3
SYMBOL MEANING
Batch code
Sterilised with ethylene oxide
Can be used until
Temperature limit
Humidity limit
Air pressure limit
Protect from moisture
Only use indoors
Fragile
Do not use if packaging damaged
Not for re-use
Dispose of in compliance with waste electronic appliances guidelines
Do not re-sterilise
Warning of cutting or penetrating injuries
JAPAN TUV R-PSE
Energy efficiency level VI
15ENMESO POWER DEVICE MS-3
SYMBOL MEANING
Device complies with Japanese VCCI standards
CHINA SJ/T 11364-2014
UL certification for components recognised in Canada and the USA
Double insulation / device protection class II
GHOST-R mark for Russia
Device complies with the requirements of RoHS 2
SIQ mark license EN60601-1
Ukraine UKRSepro
See operating manual for further information
3 SCOPE OF DELIVERY
1 Controller
1 Handpiece
1 Handpiece cable
1 Power supply
1 country-specific adapter (EU)
1 Foot switch
1 German operating manual
1 English operating manual
The needle cartridges and handpiece covers (E-0610) required for operation are not in-
cluded in the delivery. Suitable accessory parts for the handpiece can be obtained via spe-
cialist retailers, see "4.3Accessories and spare parts" on page16.

16 EN MESO POWER DEVICE MS-3
4 PRODUCT INFORMATION
4.1 Technical data
Device type AD12901
Nominal voltage 15VDC
Power consumption max. 7 VA
Power supply model Model number: GTM96180-1817.9-2.9
Part number: WR9QG1200CSPCR6B2958 (GlobTek)
Protection class 2
Penetration frequency 50-150 Hz
Drive Precision motor - DC
Operating mode Continuous operation
Dimensions (W x H x D) 230mm x 45mm x 185mm
Handpiece weight approx. 80g
Total weight approx. 1100 g
4.2 Operating conditions
Ambient temperature +10°C to +35°C
Relative humidity 30% to 75%
Air pressure 700hPa to 1070hPa
4.3 Accessories and spare parts
The following accessories and spare parts can be purchased from authorised retailers.
ACCESSORIES ARTICLE DESCRIPTION SCOPE OF
DELIVERY PIECE/VPE
Needle cartridges E-MM0002 (OND P 0.5 C VYTAL)
E-MM0006 (OND M 0.7 C VYTAL)
–
–
15
15
Handpiece cover E-0610 – 16

17ENMESO POWER DEVICE MS-3
SPARE PART ARTICLE DESCRIPTION SCOPE OF
DELIVERY PIECE/VPE
Controller MAD12901 1 1
Handpiece CMN50 1 1
Foot switch E-1010 1 1
Handpiece tray FG-AD1.0-1 1 1
Power supply E1165 1 1
Country-specific
adapter
EU: E-1154 1 1
Operating manual 7EAD12901EN
7EAD12901DE
1
1
1
1
5 GETTING STARTED
CAUTION
Cable tripping hazard
An incorrectly laid cable can constitute a tripping hazard and cause injury.
EEnsure all cables are positioned so that no one can trip over them or pull on them un-
intentionally.
CAUTION
Danger of injury and of device malfunctions
Unsuitable accessories and spare parts can impair the function and safety of the device
The device can be damaged or can fail or malfunction endangering persons.
EUse only the needle cartridges, accessories and spare parts listed in chapter
"4.3Accessories and spare parts" on page16.

18 EN MESO POWER DEVICE MS-3
ATTENTION
Danger of short-circuit
There is a risk of damage to electronics if there is visible damage to cables or cable con-
nections.
ECheck the device and the cables by visually checking for damage such as a defective
cable connection for example.
ENever kink the product's cable.
ATTENTION
Damage from condensate
If the device is exposed to high temperature variations e.g. during transport, condensate
may accumulate inside and damage the electronics.
EEnsure that the device has reached ambient temperature before using it. If the device
has been exposed to high temperature variations, wait for at least 3 hours per 10°C
of temperature difference before using it.
5.1 Positioning the device
CAUTION
Reduced functionality due to electromagnetic interference
Portable and mobile HF communications devices, such as mobile phones or WLAN
routers, can influence the functionality of the device through the transmission of electro-
magnetic radiation. Safe operation of the device can no longer be assured.
EAvoid sources of interference by ensuring that portable or mobile radio devices are not
operated in the vicinity of the device.
EInform your customers about the risk of sources of interference.

19ENMESO POWER DEVICE MS-3
ATTENTION
Damage to the device due to inadequate stability
If the device is not positioned safely, safe operation is not guaranteed. The device could
fall and get damaged during operation.
EPlace the device on a clean, firm and level surface.
EEnsure that the buttons and the display of the device remain freely accessible during
use.
ENever place the device on or underneath other devices.
EPlace the device on a clean, firm and level surface.
5.2 Connecting the power supply
The device may only be operated with the power supply cited on the type plate of the
device. The mains voltage must match with the device voltage cited on the label of the
power supply.
EIf there is no suitable country-specific adapter included in the scope of delivery, contact
an authorised dealer (see also chapter"10Questions and problems" on page30).
To remove the existing country-specific adapter, if necessary:
EPull back and hold the catch for the adapter and at the same time lift the country-spe-
cific adapter out of the recess in the power supply.
Fig.1: Remove country-specific adapter
To insert the desired country-specific adapter:
EPlace the country-specific adapter in the recess of the power supply as shown below.

20 EN MESO POWER DEVICE MS-3
EPress the adapter into the plug-in power unit until the catch can be heard to engage.
Fig.2: Inserting country-specific adapter
In order to establish the power supply:
Fig.3: Socket for the power supply
EInsert the power supply into a mains outlet.
The standby LED on the controller illuminates white.
5.3 Connecting the foot switch (optional)
Only the foot switch cited in chapter "4.3Accessories and spare parts" on page16 will en-
sure safe operation.
EInsert the RCA plug of the foot switch into the socket for the foot switch ( ) on the rear
of the controller.
Fig.4: Socket for the foot switch
This manual suits for next models
1
Table of contents
Popular Personal Care Product manuals by other brands

mychway
mychway MS-11R1 user manual

Verilux
Verilux Mood Enhancement System VT01 product manual

aidapt
aidapt VY440 Assembly and operating instructions

Forte
Forte Zephair Maintenance & Care Instruction

Physipro
Physipro PELVIC POSITIONING BELT WITH DOUBLE... owner's manual

Ahrong Eltech
Ahrong Eltech REX-KARA II user guide