Diversatek Healthcare PriZm User manual

Central Unit for Zvu®Manometry
Installation and User’s Guide
2460
Part Number: H20-0195 Rev B
Diversatek Healthcare
Technical Research & Training Center
9150 Commerce Center Circle, Suite 500
Highlands Ranch, CO 80129 USA
P800.558.6408 or 303.470.7020
DiversatekHealthcare.com

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 ii
Notes, Notices, and Cautions in User’s Guide
NOTE:
A NOTE indicates important information that helps you make better use of
your system.
NOTICE:
A NOTICE indicates either potential damage to hardware or loss of data and
tells you how to avoid the problem.
CAUTION:
A CAUTION indicates a potential for property damage, personal injury, or
death.
Symbols Marked On Devices
Refer to Instruction
Manual:
The operator must read, understand, and follow all instructions in the
accompanying documents including all warnings, cautions, and precautions
before using the medical device.
General Warning Sign:
General warning sign to alert the user to potential hazards.
Use-By Date (YYYY-
MM-DD):
Expiration date for single use and reusable catheters.
Do Not Reuse:
Marked on single use devices.
Non-Sterile:
The product associated with this symbol is not sterilized after
manufacturing.
No Pushing:
Do not push at this location. Doing so may cause the cart to overbalance
and become unstable.
EC Representative:
Authorized Representative in EU
Manufacturer:
Name and address of device manufacturer.
Serial Number:
The manufacturer’s serial number uniquely identifying the device.
Part / Reference Number:
The manufacturer’s part number of the device for re-order.
Medical Device:
Indication the device is a medical device.
Direct Current Voltage:
The type of input voltage required by the device and the voltage levels
needed.
Do Not Discard:
The device contains electronics and must be disposed of in accordance with
local regulations.

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 iii
PriZm Central Unit Classifications
Type BF Applied Part:
This symbol indicates that the patient applied part is Type BF, (floating from
electrical ground) which offers a specific level of safety.
Power Source
Classification:
Class I Equipment - Requires protective earth grounding.
Ingress Protection:
Not protected against ingress of moisture. Equipment is not suitable for use
with flammable anesthetics.
Rx Only
Prescription Only:
Device restricted for use by or on the order of a physician.
Definitions, Abbreviations and Acronyms
PriZm®:
Data acquisition system.
HRiM:
High Resolution Impedance Manometry.
LCD:
A computer monitor which uses a liquid crystal display to display the output of a computer to the user.
USB:
Universal Serial Bus. USB is a standard data I/O interface that enables the user to connect peripheral
devices to a personal computer.
Catheter (Probe)
Patient applied sensor device.

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 iv
© Copyright 2020 by Diversatek Healthcare.
All rights reserved. Reproduction in any manner whatsoever without the written permission of Diversatek
Healthcare is strictly forbidden.
DISCLAIMER: The information in this manual is subject to change without notice. Diversatek Healthcare makes
no representations or warranties with respect to the contents hereof, and specifically disclaims any implied
warranties of merchantability or fitness for a particular purpose. Diversatek Healthcare reserves the right to revise
this publication and to make changes from time to time in the content hereof without obligation of Diversatek
Healthcare to notify any person of such revisions or changes.
Trademarks used in this text: PriZm®, ZVU®, and HRiM are trademarks of Diversatek Healthcare; Dell is a
trademark of Dell Inc.; Intel, Pentium are registered trademarks of Intel Corporation; Microsoft and Windows are
registered trademarks of Microsoft Corporation.
Other trademarks and trade names may be used in this document to refer to either the entities claiming the marks
and names or their products. Diversatek Healthcare disclaims any proprietary interest in trademarks and trade
names other than its own.

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 v
Contents
1Introduction .........................................................................................................................................................1
1.1 HOW TO USE THIS GUIDE...............................................................................................................................1
1.2 CAUTION: SAFETY INSTRUCTIONS ..............................................................................................................2
1.3 PRODUCT DESCRIPTION..................................................................................................................................5
1.3.1 Indications of Use..................................................................................................................................5
1.3.2 Contraindications ..................................................................................................................................5
1.3.3 Features.................................................................................................................................................5
1.3.4 Biocompatibility.....................................................................................................................................5
2System Components ............................................................................................................................................6
2.1 USER MANUALS .............................................................................................................................................6
2.2 MAIN SYSTEM COMPONENTS .........................................................................................................................6
2.3 CONFIGURATIONS...........................................................................................................................................6
2.3.1 Cart Configuration ................................................................................................................................7
2.3.2 Desktop Configuration...........................................................................................................................8
2.4 ELECTRICAL CONFIGURATION........................................................................................................................9
2.5 ISOLATION TRANSFORMER SETUP (FOR DESKTOP CONFIGURATION)..............................................................9
2.5.1 Voltage Settings (for Desktop Configuration) .....................................................................................10
2.6 CONNECTING THE SYSTEM TO A DATA NETWORK........................................................................................11
3Controls and Connections.................................................................................................................................12
3.1 PRIZM CENTRAL UNIT..................................................................................................................................12
3.1.1 Ports, Switches and Indicators ............................................................................................................13
3.1.2 Automated Air Calibration ..................................................................................................................14
3.2SIGNAL CONDITIONING UNITS......................................................................................................................15
3.2.1 High Resolution Catheter Adapter.......................................................................................................15
3.3 SYSTEM CART ..............................................................................................................................................16
3.3.1 Adjustable Working Height..................................................................................................................16
3.3.2 Monitor Adjustments............................................................................................................................17
3.3.3 Casters and Caster Locks ....................................................................................................................18
3.3.4 Moving the Cart...................................................................................................................................19
3.4 BASIC OPERATION OF THE SYSTEM ..............................................................................................................20
3.4.1 Plugging in the System.........................................................................................................................20
3.4.2 Turning On the System.........................................................................................................................20
3.4.3 Turning Off the System ........................................................................................................................20
4Software..............................................................................................................................................................21
4.1 SYSTEM REQUIREMENTS ..............................................................................................................................21
4.2 SOFTWARE INSTALLATION INSTRUCTIONS ...................................................................................................21
4.3 SOFTWARE ACTIVATION...............................................................................................................................22
4.4 SOFTWARE UPGRADES .................................................................................................................................22
4.5 SECURITY AND AUTHENTICATION................................................................................................................22
5Cleaning and Preventative Maintenance.........................................................................................................23
5.1 CLEANING PROCEDURE ................................................................................................................................23
5.1.1 PriZm, Cart, and Accessories..............................................................................................................23
5.1.2 Calibration Tubes................................................................................................................................23
5.1.3 Probe Case Cleaning...........................................................................................................................23
5.2 AIR CALIBRATION TUBE SEALS....................................................................................................................24
5.3 PREVENTATIVE MAINTENANCE....................................................................................................................25
5.4 SERVICE .......................................................................................................................................................25
5.5 DECOMMISSIONING AND DISPOSAL ..............................................................................................................25
6Appendix ............................................................................................................................................................26

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 vi
6.1 TECHNICAL SUPPORT ...................................................................................................................................26
6.2 DECLARATION OF CONFORMITY...................................................................................................................27
6.3 EMC INFORMATION .....................................................................................................................................28
6.3.1 Electromagnetic Emissions..................................................................................................................28
6.3.2 Electromagnetic Immunity...................................................................................................................29
6.3.3 Recommended Separation Distances...................................................................................................31
6.4 SPECIFICATIONS ...........................................................................................................................................32
6.4.1 PriZm Central Unit..............................................................................................................................32
6.4.2 Isolation Transformer..........................................................................................................................32
6.4.3 Cart......................................................................................................................................................32

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 1
1 Introduction
1.1 How to Use This Guide
This guide is designed to help you install the PriZm®System and the Zvu®software quickly and easily. It is
intended for health-care professionals trained in performing clinical procedures. Regularly scheduled clinical
training courses are offered for your convenience. See contact information on the cover page or in Section 6.1:
Technical Support.
Caution: The assembly and installation of a rolling cart system must be performed by a
trained manufacturer’s representative since some assembly is required.
For detailed instructions on using the PriZm System and Zvu Software refer to the help screens incorporated into the
software.
This User’s Guide assumes the user has the following basic computer skills common to Microsoft software
applications:
•Mouse click and double-click. If using a touch screen, then touching the screen is the same as a mouse click and
touching the screen twice in rapid succession in the same spot is the same as a double-click.
•Open desktop folders and double-click desktop icons to invoke applications.
•Use Windows Explorer to browse and manage files and folders.
•Maximize, minimize, resize, and move application windows.
•Use dialog boxes and message boxes.
•Use menu bars to execute menu commands.
This guide uses visual clues and typographical conventions to attract attention to and clarify instructions.
Keyboard keystrokes are written in bold face.
Labels in the software such as a menu, toolbar, button, shortcut names, etc. are written in italics.
The guide is divided into different sections featuring specialized tasks for quick, easy reference.

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 2
1.2 CAUTION: Safety Instructions
The PriZm®System and the accompanying signal conditioning devices are sensitive electronic instruments. Please
use the following safety guidelines to help ensure your own personal safety and to help protect your PriZm System
and working environment from potential damage.
CAUTION: The user must be qualified in gastrointestinal diagnostic procedures, trained in the use of
the system, and must be familiar with all labeling and instruction for use associated with
the equipment. Many device injuries are due to user error and failure to follow the
instructions for use. The user of the device is advised to thoroughly understand the use
of the equipment, and familiarize themselves with the location and function of all
controls and alarms prior to using the equipment.
CAUTION: The PriZm System is intended for use by gastroenterologists, surgeons, other trained
physicians, and medically trained personnel as an aid in documenting and diagnosing
digestive disorders. This system includes analysis software, but requires skilled
interpretation by a physician to make a diagnosis.
CAUTION: The assembly and installation of a rolling cart system must be performed by a trained
manufacturer’s representative.
CAUTION: Do not get the PriZm System or signal conditioning devices wet - these devices are not
waterproof.
CAUTION: Plug the PriZm System into voltages as stated on the Central Unit Nameplate.
CAUTION: Warning: To avoid the risk of electric shock, this equipment must only be connected to a
supply mains with protective earth ground.
CAUTION: For Desktop Systems: The PriZm System must receive mains power from a medical
grade isolation transformer. The isolating transformer is specified as a part of the
Medical Electrical System.
CAUTION: For Desktop Systems: Warning: Connecting electrical equipment to multiple socket
outlets effectively leads to creating a Medical Equipment System, and can result in a
reduced level of safety. Do not use the socket outlets for other devices that are not part
of the PriZm System.
CAUTION: For Desktop Systems: Do not plug another multiple socket outlet power strip into the
accessory outlets provided with the isolation transformer. Only the devices provided
with the PriZm System should be powered through the isolation transformer.
CAUTION: Do not attempt to open or service the PriZm System or signal conditioning devices.
There are no user serviceable parts inside.
CAUTION: Warning: No modification of this equipment is allowed.
CAUTION: Follow instructions provided with all types of catheters used with the PriZm System and
Signal Conditioning devices.
CAUTION: Discard all used disposable catheters in accordance with local biohazard requirements.
Refer to section 5.5 Decommissioning and Disposal for additional information.
CAUTION: Reusable catheters should be cleaned and disinfected according to manufacturer’s and
your institution’s guidelines after each use.

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 3
CAUTION: Dispose of the PriZm System and signal conditioning device in accordance with local
ordinances and regulations. Refer to section 5.5 Decommissioning and Disposal for
additional information.
CAUTION: Electromagnetic interference is possible between impedance (Z) catheters and implanted
devices such as pacemakers and internal defibrillators. Monitoring of all implanted
devices is advised.
CAUTION: Inspect the catheter for damage such as bent or broken pins. Also, verify the integrity of
the Catheter between the tubing and each sensor. Do not connect the Catheter to the
equipment if there is visible damage. Refer to the Catheter manufacturer’s cautions and
warnings found in the Catheter manufacturer’s user guide.
CAUTION: In order to minimize the risk of nosebleed, use adequate lubrication with a water soluble
lubricant for catheter intubation.
CAUTION: To clean the monitor, use a cloth that has been slightly dampened in a solution of warm
water and mild detergent. Avoid solvents which may damage the product cases. Follow
hospital protocol. Do not apply liquid directly to monitor.
CAUTION: Only use Diversatek Healthcare approved accessories with the PriZm System. Damage
to the system, the accessory, and/or patient injury may occur.
CAUTION: Do not use Diversatek Healthcare accessories with other non-Diversatek Healthcare
equipment. Damage to the system, the accessory, and/or patient injury may occur.
CAUTION: Do not use the PriZm System in association with an MRI machine. The PriZm System
contains sensitive electronics not designed to operate in the extensive magnetic fields of
an MRI machine.
CAUTION: Do not use the PriZm System or other signal conditioning devices in emergency
situations or for patient treatment or monitoring. The system is designed for diagnostic
use only in non-emergency situations.
CAUTION: Do not use the PriZm System in an oxygen rich environment.
CAUTION: Any serious incidents that occur in relation to the PriZm System should be reported to
Diversatek and the Competent Authority.
NOTICE: Use of non-Diversatek Healthcare approved USB devices may cause unpredictable
intermittent device operation.
NOTICE: Use of an uninterruptible power system is suggested if the quality of power is questionable.
NOTICE: Do not store the PriZm System or signal conditioning devices in extreme temperatures.
The PriZm System and signal conditioning devices are best stored between 33° and 158°F
(1° to 70°C).
NOTICE: Do not drop the PriZm System or the signal conditioning devices.
NOTICE: Do not run other software, update software or the operating system, or add/remove
peripherals during data acquisition.

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 4
NOTICE: Microsoft Windows does not allow the Hibernate, System Standby, or Hard Disks Power-
Off features to be deactivated through software, so Zvu®cannot deactivate these features.
Turning on any of these features may cause the termination of acquired data while the
items are shut down. Data loss may occur. Use caution when turning on these features.

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 5
1.3 Product Description
1.3.1 Indications of Use
The PriZm®Gastrointestinal Motility System is intended for use by gastroenterologists, surgeons, and medically
trained personnel as an aid in documenting and diagnosing digestive motility disorders. It may be used for
esophageal and anorectal studies. The system includes analysis software, but requires a skilled interpretation by a
physician to make a diagnosis.
1.3.2 Contraindications
Esophageal manometry is contraindicated in the following situations:
•Suspicious or known pharyngeal or upper esophageal obstruction (e.g., tumors)
•Patients with severe clotting disorders
•Patients with known esophageal problems such as deep ulcers, varices, Zenker's diverticula, and strictures
Anorectal manometry is contraindicated in the following situations:
•Patients with known anal obstructions.
Small bowel manometry is contraindicated in the following situations:
•Those associated with esophagogastroduodenoscopy (EGD).
•Massively dilated small bowel is a relative contraindication due to risk of perforation.
•Known multiple jejunal diverticulosis.
1.3.3 Features
The PriZm System was designed with the end user in mind. The PriZm System offers the following features:
•Specialized modules focused on specific clinical procedures.
•Guided Protocols to guide the operator step by step through the procedure.
•On-screen Help buttons accessible by users at any time during a procedure if detailed instructions are needed.
•Compatible with a flat panel LCD display to save space and facilitate transportation.
•Works with a touch screen display that may eliminate the need for a keyboard and mouse during data
acquisition.
•A flexible system that supports various configurations to meet the clinician’s requirements.
1.3.4 Biocompatibility
The PriZm System components utilize common materials with no known biocompatibility issues. However, the
following cautions should be observed:
CAUTION: Some catheters have applied parts made with 316L stainless steel. This
type of stainless steel, although medical grade, contains 10-14% nickel
which may pose risks for people with certain allergies to nickel.

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 6
2 System Components
The following sections describe the various components of the PriZm®System.
2.1 User Manuals
All of the user manuals for the PriZm System are provided in electronic versions that are installed along with the
Zvu®Software suite. The following table lists the various manuals provided. Please refer to these manuals for
additional information.
Manual Part Number
Title
H20-0195
PriZm®System Installation and User’s Guide
MI-0195
MiVu®Instructions for Use
2.2 Main System Components
The PriZm System consists of the following main components. Optional signal conditioning units are available.
Refer to the manuals accompanying those units for additional information.
2.3 Configurations
The PriZm System is available in two configurations: Cart and Desktop. The active medical components are the
same for both the cart and desktop configurations. The rolling cart is offered for those customers who require
additional mobility.
PriZm Central Unit:
Provides patient isolation for safety; translates data format and
transfers data to host computer.
Host Computer:
Provides computer processing capabilities to acquire, store, and
analyze recorded patient data.
Touch Screen Monitor:
The display shows the waveform data that is being acquired from the
patient.
Catheters and Transducers:
Patient applied parts to collect biomedical data and convert it into a
format that can be displayed and analyzed using a computer.
Isolation Transformer:
Safety device required for use with system. Provides electrical
isolation of the system and minimizes potential leakage current.
Printer:
Optional accessory for producing hard copy reports.

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 7
2.3.1 Cart Configuration
The PriZm®System is offered with an optional rolling cart. The following diagram shows the locations of the main
components.
Printer
PC
PriZm
Central Unit
Keyboard
and Mouse
Tray
Air
Calibration
Tube
Water
Calibration
Tube
Touch
Screen
Monitor
Storage
Compartment
AC Mains
Power Switch
PriZm
Central Unit
Height
Adjustment
Pedal
AC
Mains
Power
Entry
Catheter
Cable Holder
Isolation
Transformer
(under cover)

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 8
2.3.2 Desktop Configuration
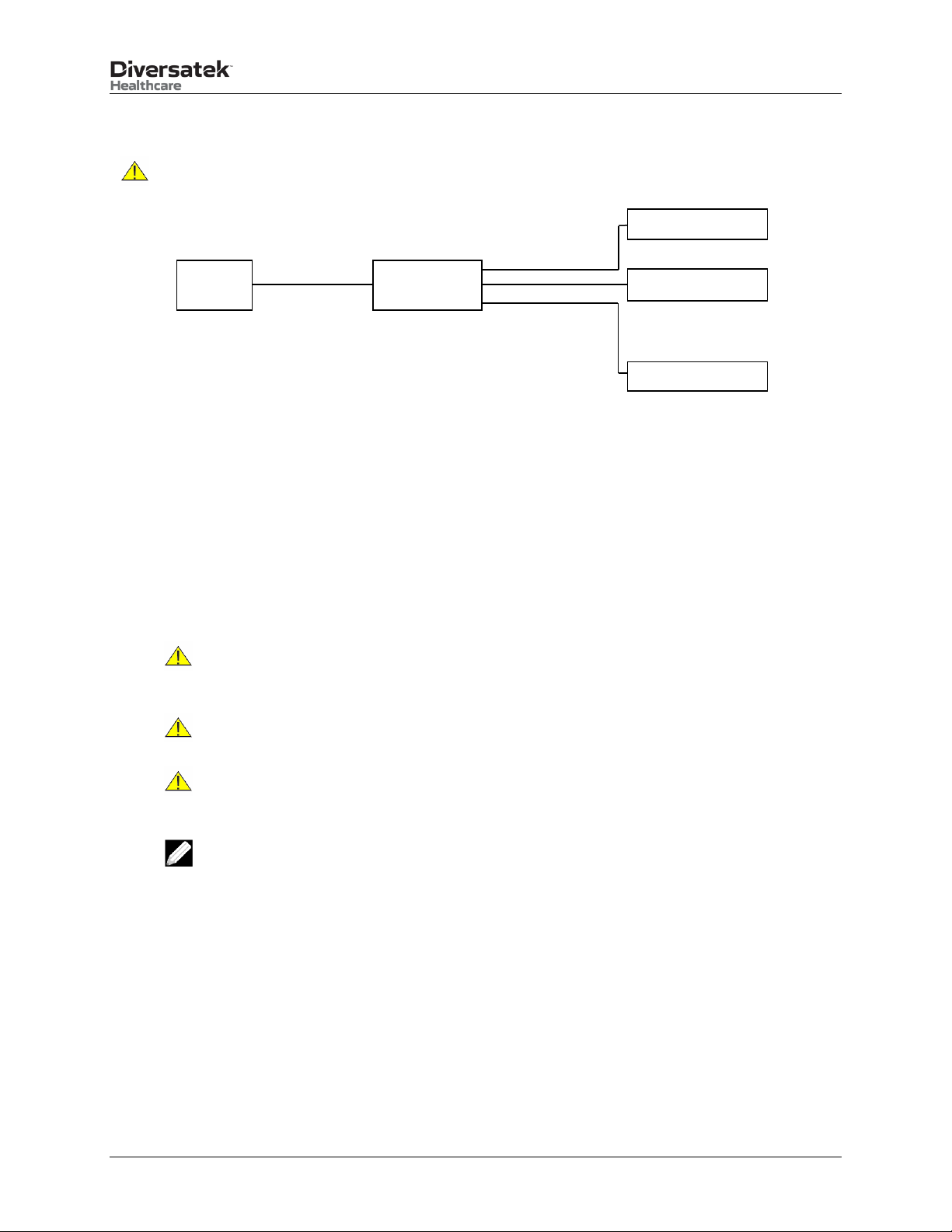
The PriZm®System is offered in a desktop configuration. The following diagram shows the main components.
PC
Touch Screen Monitor
Isolation
Transformer
PriZm
Central Unit
Items not shown:
•Water Calibration Tube
•Air Calibration Tube
•Hand-held Pressure Gauge
•Printer
PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 9
2.4 Electrical Configuration
CAUTION: A suitable medical grade isolation transformer is
required for this system.
Figure 1: Electrical Configuration
Connect the system components together according to the following manner (refer to Figure 1 above):
•Connect all AC power cords to the isolation transformer.
2.5 Isolation Transformer Setup (for Desktop Configuration)
An isolation transformer is a critical safety device that helps to isolate the patient from electrical hazards. The
following precautions must be followed for the isolation transformer to provide the best protection possible.
CAUTION: The PriZm System must receive AC Mains power from a medical grade isolation
transformer. The supplied isolating transformer is specified as a part of the
Medical Electrical System.
CAUTION: Connect all devices of the PriZm System to the output side of the isolation
transformer as described in section 2.4 above.
CAUTION: Do not connect any devices not associated with the PriZm System to the output
side of the isolation transformer. The isolation transformer should only be used
with the PriZm System components.
See the appendix for the isolation transformer specifications.
Isolation
Transformer
AC
Mains
LCD Monitor
Host Computer
PriZm

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 10
2.5.1 Voltage Settings (for Desktop Configuration)
The Input and Output voltage settings must be configured before connecting the isolation transformer into the AC
Mains power source. The Input voltage selector is set through a four-position key that is located within the fuse
holder of the power entry module (see below). When setting the voltage selector, the desired voltage will be visible
in the window. The Output voltage selector is set through a two-position switch on the bottom of the unit (see
below). Refer to the following table for the proper Input and Output voltage settings for your region. If your input
voltage for your area is not listed below, please contact Technical Support for assistance.
Region
Input
Output
North America (120 V~)
120 V~
115 V~
Europe (230-240 V~)
240 V~
230 V~
Japan (100 V~)
100 V~
115 V~
Other 220 V~
220 V~
230 V~
Input Voltage
Selector
Output
Voltage
Selector

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 11
2.6 Connecting the System to a Data Network
The PriZm®System does not need to be connected to a data network for it to function properly and achieve its
intended use. Attachment to a network provides for convenient sharing of data files, back-up of data, and printing to
network printers. There are no known hazardous situations resulting from a failure of the network coupling.
The IT data network port utilized by the PriZm System conforms to the conventional Ethernet standard IEEE 802.3.
There are no required characteristics or configuration of the IT network.
If the PriZm System is connected to a data network, please be aware of the following:
CAUTION: Connection of the PriZm System to a network/data coupling that
includes other equipment could result in previously unidentified risks
to patients, operators or third parties. All risks should be identified,
analyzed, evaluated and controlled. Subsequent changes to the
network/data coupling could introduce new risk and require
additional analysis such as unauthorized access. Changes to the
network/data coupling include:
•Changes in network/data coupling configuration
•Connection of additional items to the network/data coupling
•Disconnecting items from the network/data coupling
•Update of equipment connected to the network/data coupling
•Upgrade of equipment connected to the network/data coupling
Please refer to the current revision of IEC 60601-1 for the requirements applicable to Medical Electrical (ME)
Systems.

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 12
3 Controls and Connections
3.1 PriZm Central Unit
The PriZm®Central Unit is the main component of the PriZm system. It provides data buffering to the acquisition
software running on the PC.
Accessory Port 4
Air Outlet
Air Inlet
DC Power Input
USB
Port 5
(unused)
Port 1 –High
Resolution Catheters
Accessory Port 2
ISO Power Indication LED
and Port 1-5 Status LEDs
Power Switch and
Power Indication LED
Accessory Port 3

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 13
3.1.1 Ports, Switches and Indicators
•USB - Connection port for the USB cord attaching the system to the PC.
•Power Switch –This controls the DC power supplied by the external medical grade power supply brick.
Note this switch controls the power supplied to the
PriZm®Central Unit and all attached signal conditioning
units.
•DC Power Input –This is the DC power input receptacle.
CAUTION: Warning: To avoid the risk of electric shock, this equipment must
only be connected to an AC Mains with protective earth ground.
CAUTION: The PriZm System must receive AC Mains power from a medical
grade isolation transformer. The supplied isolating transformer is
specified as a part of the Medical Electrical System.
•Air Inlet and Outlet Ports –Air supply and pressure sensing ports for the automated calibration tube
feature.
•Port 1 –A patient isolated connection port for attaching to the High Resolution Catheters.
•Ports 2-4 –Non-patient isolated Accessory Ports for attaching external device adapters. Patient
connected external device adapters have their own built-in patient isolation.
•Port 5 –Unused. Reserved for future expansion.
•Power Indication LED –A green LED that is illuminated when the Power Switch is in the ON position
and the DC input is measured to be at the appropriate voltage levels.
•ISO Power Indication LED –A green LED that is illuminated when the isolated power of the internal
High Resolution Catheter Adapter board is determined to be at the appropriate levels for
operation.
•Port 1 Indication LED –A blue LED that is illuminated when the High Resolution Catheter is attached
to Port 1 and communicating properly.
•Port 2-4 Indication LEDs –Blue LEDs that are illuminated when an external device adapter module is
plugged in and communicating properly. These LEDs will blink if the PriZm Central Unit
detects the device adapter module was plugged in but communication was not established. If
blinking, try re-plugging in the device or use a different port. Contact Technical Support for
assistance.

PriZm®System
Installation and User’s Guide
H20-0195 Rev B, Edited 12/10/2020, ECO 220-034 14
3.1.2 Automated Air Calibration
The Zvu®Software is able to utilize the automated air calibration feature of the PriZm®System. Inside the PriZm is
a small air pump that Zvu turns on to pressurize the air calibration tube. Zvu then monitors the PriZm’s calibration
sensor to detect the calibration points. The releasing of the air is accomplished through two tiny vent holes, one
located in each of the two air lines.
To use the automated calibration feature of the PriZm two air lines must be installed between the PriZm and the air
calibration tube cap.
a) Disconnect the sphygmomanometer from the air calibration tube. To release
the fitting, press down on the metal tab of the port on the calibration tube.
b) Connect the straight fitting ends of the two air lines to the two ports on
the PriZm. The air lines are identical and can be plugged into either
port. The fittings are fully seated when the metal tab on the port clicks
up.
c) Connect the elbow fitting of the two tubes to the two ports on the air
calibration tube. The air lines are identical and can be plugged into either
port. Twist the fittings so the barbed end of the fitting points upward and
the tubing is not kinked.
To perform a manual calibration, disconnect both connectors from the air calibration tube only. Leave the air lines
connected to the PriZm. Connect the sphygmomanometer to either of the connector ports on the air calibration tube.
Table of contents

















