Technical
Informat
ion:
Toll
free
1-
800-606-6246, or 1-760-929-9911 Email: technica[email protected]m Website: www.mobio.com 6
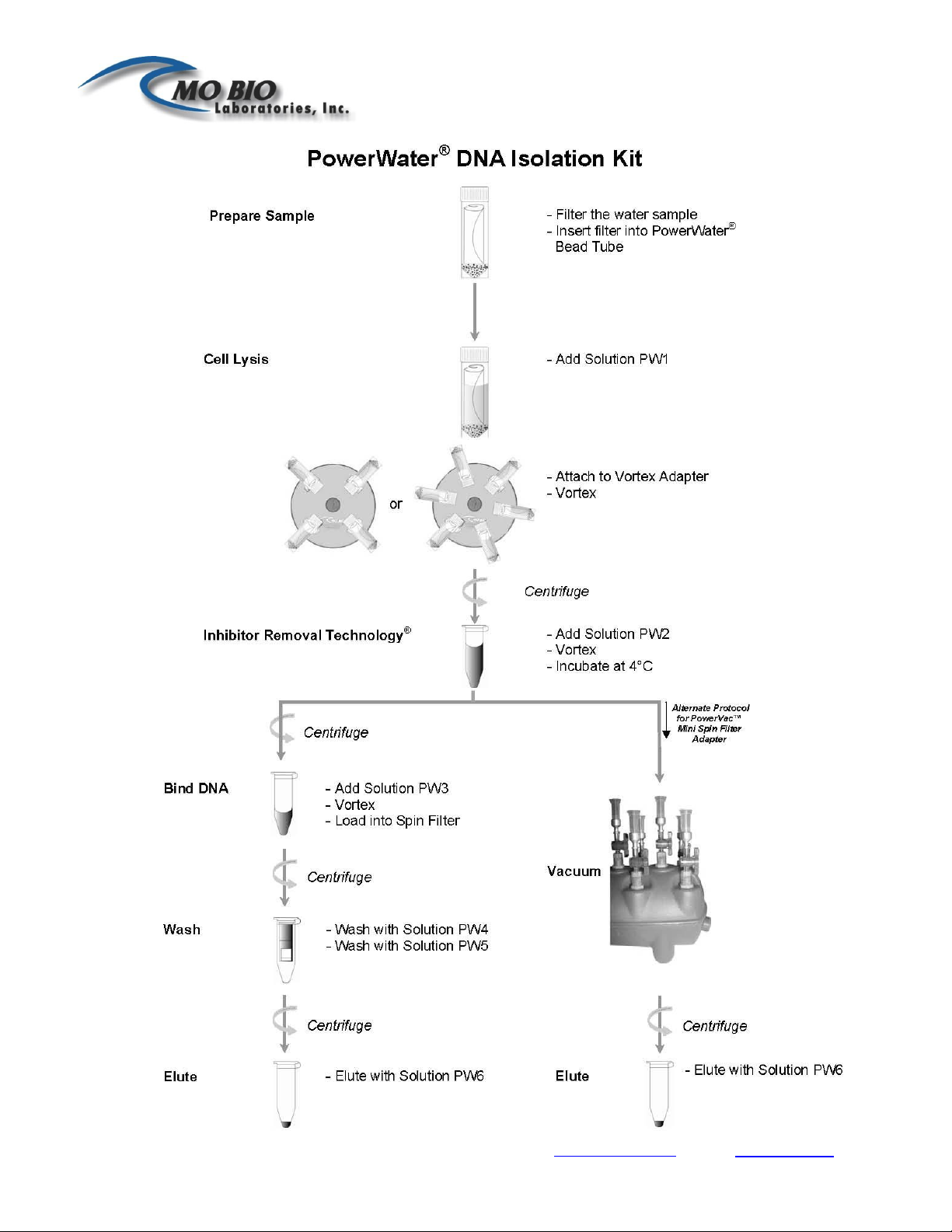
Experienced User Protocol
Please wear gloves at all times
Warm Solution PW1 prior to use at 55°C for 5-10 minutes. Use Solution PW1 while still warm.
Check Solution PW3 and warm at 55°C for 5-10 minutes if necessary. Solution PW3 can be used
while still warm.
1. Filter water samples using a reusable or disposable filter funnel attached to a vacuum source.
Disposable filter funnels, containing 0.22 µm or 0.45 µm filter membranes, can be ordered from
MO BIO Laboratories (see page 3). The volume of water filtered will depend on the microbial
load and turbidity of the water sample. (Please see Types of Water Samples in the Hints and
Troubleshooting Guide section of the Instruction Manual).
2. If using a reusable filter funnel, remove the upper portion of the apparatus. If using a MO BIO
Laboratories filter funnel, remove the 100 ml upper portion of the filter cup from the catch
reservoir by snapping it off.
3. Using two sets of sterile forceps, pick up the white filter membrane at opposite edges and roll the
filter into a cylinder with the top side facing inward.
Note: Do not tightly roll or fold the filter membrane. To see a video of this technique,
please visit the PowerWater® DNA Isolation Kit product page on www.mobio.com.
4. Insert the filter into the 5 ml PowerWater®Bead Tube.
5. Add 1 ml of Solution PW1 to the PowerWater®Bead Tube.
Note: Solution PW1 must be warmed to dissolve precipitates prior to use. Solution PW1
should be used while still warm. For samples containing organisms that are difficult to
lyse (fungi, algae) an additional heating step can be included. See Alternate Lysis
Method in the Hints and Troubleshooting Guide.
6. Secure the PowerWater®Bead Tube horizontally to a MO BIO Vortex Adapter, catalog number
13000-V1-15 or 13000-V1-5.
7. Vortex at maximum speed for 5 minutes.
8. Centrifuge the tubes ≤ 4000 x g for 1 minute at room temperature. The speed will depend on the
capability of your centrifuge. (This step is optional if a centrifuge with a 15 ml tube rotor is
not available, but will result in minor loss of supernatant).
9. Transfer all the supernatant to a clean 2 ml Collection Tube (provided). Draw up the supernatant
using a 1 ml pipette tip by placing it down into the beads.
Note: Placing the pipette tip down into the beads is required. Pipette more than once to
ensure removal of all supernatant. Any carryover of beads will not affect subsequent
steps. Expect to recover between 600-650 µl of supernatant depending on the type of
filter membrane used.
10. Centrifuge at 13,000 x g for 1 minute.
11. Avoiding the pellet, transfer the supernatant to a clean 2 ml Collection Tube (provided).
12. Add 200 µl of Solution PW2 and vortex briefly to mix. Incubate at 4°C for 5 minutes.
13. Centrifuge the tubes at 13,000 x g for 1 minute.
14. Avoiding the pellet, transfer the supernatant to a clean 2 ml Collection Tube (provided).
15. Add 650 µl of Solution PW3 and vortex briefly to mix.
Note: Check Solution PW3 for precipitation prior to use. Warm if necessary. Solution
PW3 can be used while still warm.
16. Load 650 µl of supernatant onto a Spin Filter and centrifuge at 13,000 x g for 1 minute. Discard
the flow through and repeat until all the supernatant has been loaded onto the Spin Filter.
Note: A total of two loads for each sample processed are required.
17. Place the Spin Filter basket into a clean 2 ml Collection Tube (provided).