3 MTS_OW100_IFU-orthogold100-US-K182682_A
Tables
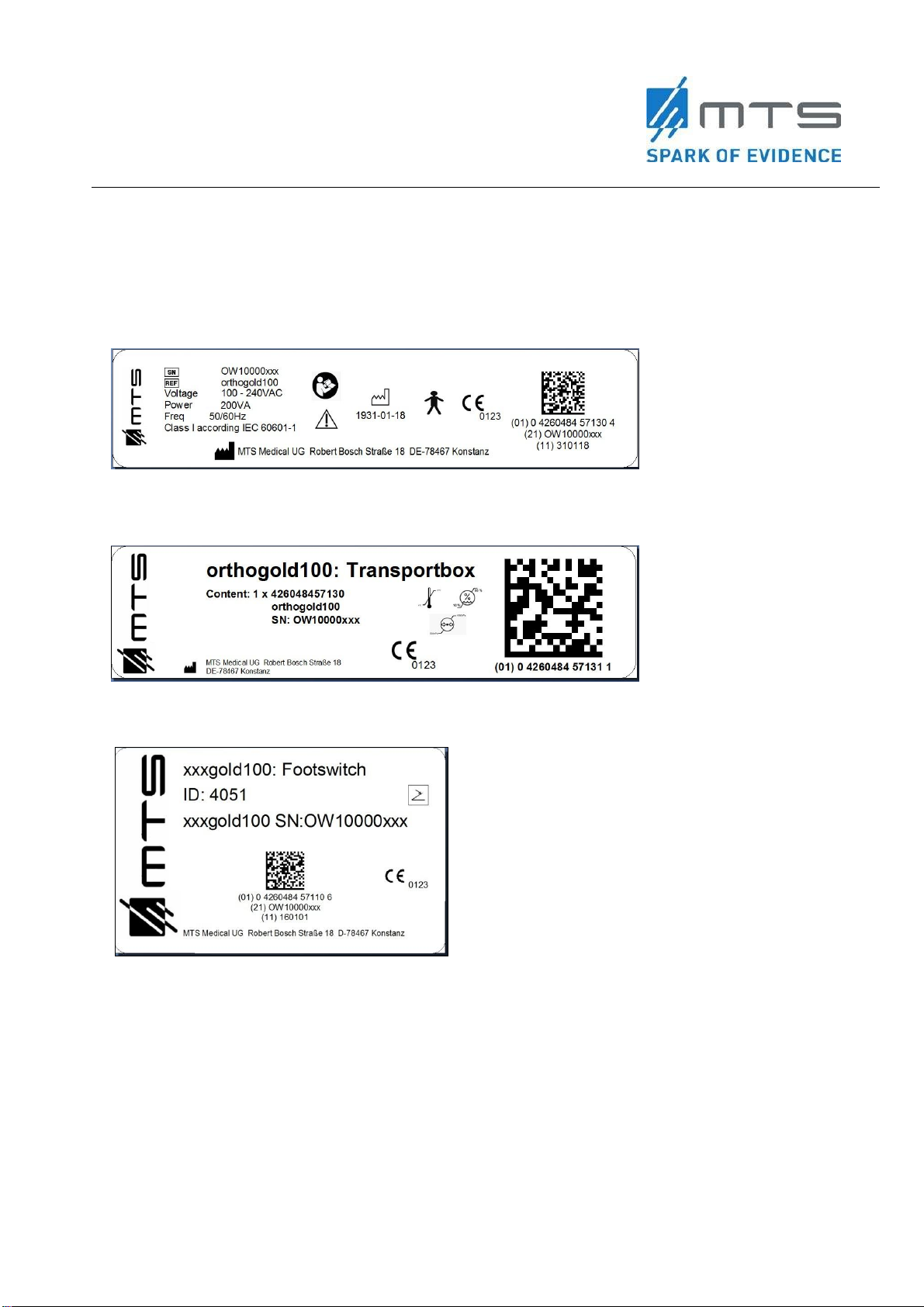
Table 1: List of delivered parts with the OW100®..................................................................................... 21
Table 2 Recommended safety distances between Mobile HF Telecommunication Devices and the
OW100®.................................................................................................................................................. 57
Table 3 Electromagnetic Interference...................................................................................................... 59
Table 4 Resistance to Electromagnetic Interference ............................................................................... 60
Table 5 Guidance and manufacturer´s declaration.................................................................................. 61
Table 6 Troubleshooting Issues .............................................................................................................. 64
Figures
Figure 1
Function Principle of acoustic wave creation with the OW100
®
............................................ 11
Figure 2
Components and connections of the OW100
®
at the front side
............................................ 12
Figure 3
Components and connections of the OW100
®
at the rear side
............................................. 13
Figure 4
Applicator
................................................................................................................................. 14
Figure 5
Water cartridge of the OW100
®
at the rear side
..................................................................... 15
Figure 6
Front side connections of the OW100
®
................................................................................... 16
Figure 7
Connections at the rear side
.................................................................................................... 17
Figure 8
Applicator connection socket (left) and connect applicator (right)
......................................... 18
Figure 9
Display of the OW100
®............................................................................................................ 18
Figure 10
Touch panel (left) and touch wheel (right) of the OW100
®
................................................... 19
Figure 11
Footswitch
.............................................................................................................................. 19
Figure 12 Installation of power supply..................................................................................................... 22
Figure 13
Applicator connection - inside view (left) and socket (right)
................................................. 24
Figure 14
Connected applicator
............................................................................................................. 24
Figure 15
Display of the connected applicator at the OW100
®
............................................................ 24
Figure 16 Applicator drain control............................................................................................................ 28
Figure 17
Pit for water cartridge (1)
....................................................................................................... 29
Figure 18
Unlocked water cartridge
....................................................................................................... 29
Figure 19
Inserted and locked water cartridge (2)
................................................................................ 29
Figure 20
Water cartridge of the OW100
®
shown from above (left) and from front side (right)
.......... 30
Figure 21
Set-up Menu of the OW100
®
................................................................................................. 31
Figure 22
Connected Footswitch
........................................................................................................... 31
Figure 23
"SETUP' Key field
.................................................................................................................. 32
Figure 24
Power switch activated
.......................................................................................................... 37
Figure 25
Step 3 - when to press the confirmation button
.................................................................... 37
Figure 26
Start screen of the OW100
®
with displayed software version
.............................................. 37
Figure 27
Operation screen
................................................................................................................... 38
Figure 28
"FILL" key on the display
....................................................................................................... 39
Figure 29
Touch panel of the OW100
®
.................................................................................................. 44
Figure 30
Touch wheel of the OW100
®
................................................................................................. 45
Figure 31
Options for "ENERGY" on the display
.................................................................................. 45
Figure 32
Beam pressure maximum and target Location
..................................................................... 46
Figure 33 Dependence of the pressure pulse parameters on the generator............................................ 47
Figure 34 Dependence of the acoustic pulse energy on the generator.................................................... 48
Figure 35
Dependence of the pressure pulse parameters on the generator
....................................... 48
Figure 36
Setting the frequency at the display
...................................................................................... 49
Figure 37
Pre-set of the number of shots for one treatment
................................................................. 50
Figure 38
Display of the connected applicator types at the display of the OW100
®
............................ 50
Figure 39
Display of the remaining acoustic waves on the applicator
................................................. 51