HOW TO USE WIXELATM INHUBTM
Wixela Inhub was designed with patients in mind to help you make
a seamless transition from ADVAIR DISKUS®(fluticasone propionate
and salmeterol inhalation powder).
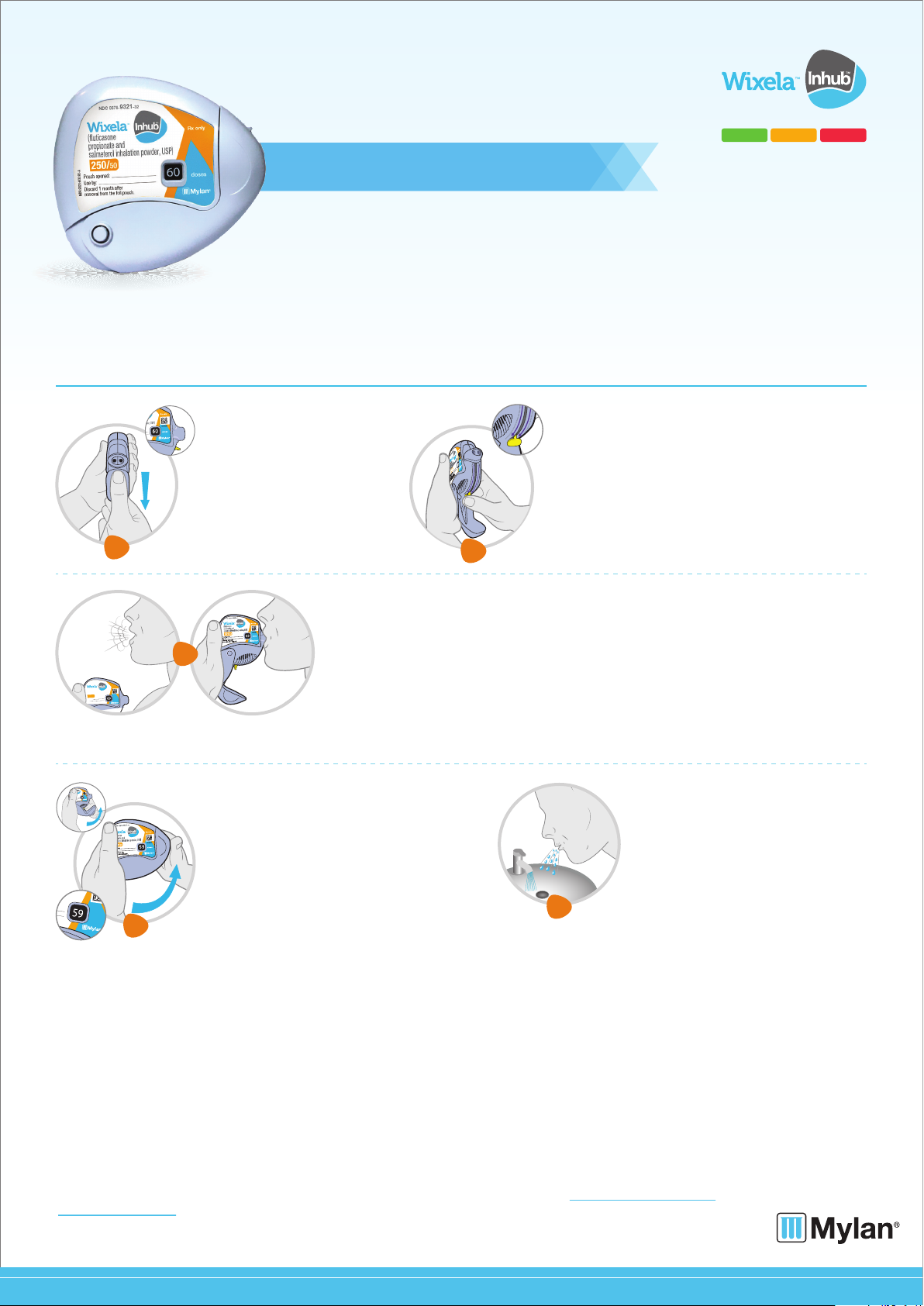
Take Wixela Inhub out of the foil pouch just before you use it for the first time. Write the “Pouch opened” and “Use by” dates on the label.
The “Use by” date is one month from the date you open the pouch for your first dose.
(fluticasone propionate
and salmeterol inhalation powder, USP)
100/50 mcg250/50 mcg 500/50 mcg
Follow the 5 steps below each time you take a dose.
12
• Hold the INHUB in one hand
and with your other hand on the
grip lower the mouthpiece
cover from top to bottom.
Open your INHUB
• Hold the INHUB in the vertical position. Push the
yellow lever down to the end of the purple arrows (you
may hear a click).
• The INHUB is now ready to use.
Follow the instructions below so you will not accidentally
waste a dose:
•Do not close the INHUB.
•Do not move the lever on the INHUB once pushed down.
Push down the lever.
4
doses
00000000
Discard 1 month after
removal from the foil pouch.
(fluticasone
propionate and
salmeterol inhalation powder, USP)
doses
00000000
Rxonly
Pouchopened:
Useby:
Discard1monthafter
removalfromthefoil pouch.
MDR:9320:60D:R2-A
NDC0378-
9320
-32
(fluticasone
propionateand
salmeterolinhalation powder, USP)
100/
50
5
• Push the mouthpiece cover up to the closed
position, this will automatically return the yellow
lever to the start position. Make sure the INHUB
is shut and you cannot see the mouthpiece.
• The dose counter will count down one dose as
you close the mouthpiece cover. This will now
tell you how many doses are left.
• The INHUB is now ready for you to take your
next scheduled dose in about 12 hours.
•When you are ready to take your next dose,
repeat Steps 1 through 4.
Close the INHUB
•Rinse your mouth with water after
breathing in the medicine. Spit out the
water. Do not swallow it.
Rinse your mouth
doses
00000000
Rxonly
Pouchopened:
Useby:
Discard1month after
removalfrom the foil pouch.
A-2R:D06:0239:RDM
NDC0378-
9320
-32
(fluticasone
propionate and
salmeterol inhalation powder, USP)
250/
50
• Before you breathe in your dose from the INHUB, breathe out (exhale) as long as you
can while you hold the INHUB away from your mouth. Do not breathe into the mouthpiece.
• Put the mouthpiece to your lips. Breathe in quickly and deeply through the INHUB.
Do not breathe in through your nose.
• Remove the INHUB from your mouth and hold your breath for about 10 seconds,
or for as long as is comfortable for you.
•Breathe out slowly for as long as you can.
• The INHUB delivers your dose of medicine as a very fine powder that you may or may not taste
or feel. Do not take an extra dose from the INHUB even if you do not taste or feel the medicine.
Inhale your medicine.
3
Indications
WIXELA INHUB is a twice-daily prescription medicine used to control symptoms of asthma and to prevent symptoms such as
wheezing in patients 4 years and older. WIXELA INHUB is for adults and children with asthma who are not well controlled with an
asthma control medicine, such as an inhaled corticosteroid (ICS) medicine and need both an ICS and long-acting beta2-adrenergic
agonist (LABA) medicine.
WIXELA INHUB 250/50 is a twice-daily prescription medicine used long term to treat chronic obstructive pulmonary disease
(COPD), including chronic bronchitis, emphysema, or both, for better breathing and fewer flare-ups.
Important Limitation of Use: WIXELA INHUB is not used to relieve sudden breathing problems from asthma or COPD and won't
replace a rescue inhaler.
Please see additional Important Safety Information on next page. Click here for full Prescribing Information and
Patient Information for full Instructions for Use.

