Neural Analytics NeuralBot NA-RBT1 User manual

NeuralBot™ System User Manual (60-00119, Rev. A)
1
NeuralBot™System
For use with the Lucid™M1 System
User Manual
Copyright © 2019 By Neural Analytics, Inc.

NeuralBot™ System User Manual (60-00119, Rev. A)
2
NeuralAnalytics,Inc.All rights reserved. No part of this manualmaybereproduced,storedinaretrievalsystem,or transmitted, in any
form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of
Neural Analytics.
Year of Authorization: 2018
COMPANY DATA Neural Analytics, Inc.
2440 S. Sepulveda Blvd, Suite 115
Los Angeles, CA 90064, USA
Phone: 877-638-7251 ext. 733
Email: support@neuralanalytics.com
AUTHORIZED REPRESENTATIVE
Healthlink Europe Services BV
De Tweeling 20-22
5215 MC’s-Hertogenbosch
The Netherlands
Tel Nr: +31.13.5479300
Fax Nr: +31.13.5479301
E-mail: complaints@HealthlinkEurope.com
PRODUCT INFORMATION NeuralBot™™
MODEL NA-RBT1 (NeuralBot™)

NeuralBot™ System User Manual (60-00119, Rev. A)
3
Table of Contents
Introduction ..................................................... 5
How to Use this Manual ...................................................................................5
Indications for Use ...........................................................................................5
Contraindications.............................................................................................5
Intended Use Environment..............................................................................5
Compatibility.....................................................................................................5
Conventions used in this Manual....................................................................6
Symbols.............................................................................................................7
Product Description......................................... 8
Overview of the NeuralBot™ System .............................................................8
Package Contents ..........................................................................................10
Cautions and Warnings..................................................................................11
Clinical Safety.................................................................................................13
Ultrasound Safety...........................................................................................13
Operating NeuralBot™ ................................... 14
Overview of the NeuralBot™ procedure.......................................................14
System Pre-Checks........................................................................................14
Patient Setup...................................................................................................17
Registration: ...................................................................................................21
Perform Exam.................................................................................................24
Post Exam Procedure ....................................................................................25
Quick Release Procedure ..............................................................................26
System Power Down ......................................................................................26
Device Storage................................................................................................26
Export Log Files .............................................................................................27
Equipment Care and Maintenance ................ 28
Cleaning ..........................................................................................................28
Preventative Maintenance..............................................................................29
Regulatory Statements.................................. 30
Instructions on Disposal................................................................................30
Electro-Magnetic Compatibility (EMC)..........................................................30
Potential Equalization ....................................................................................31

NeuralBot™ System User Manual (60-00119, Rev. A)
4
Service and Ordering Information ................. 32
Product Reference Numbers.........................................................................33
Appendix A..................................................... 34
Quick Start Guide ...........................................................................................34
Appendix B..................................................... 36
Troubleshooting Guide..................................................................................36
Appendix C..................................................... 42
Technical Information ....................................................................................42
Appendix D..................................................... 43
ACU Display Descriptions .............................................................................43
Basic Features in the ACU Interface.............................................................44
Advanced Features in the ACU interface .....................................................46
Appendix E ..................................................... 48
Acoustic Output..............................................................................................48

NeuralBot™ System User Manual (60-00119, Rev. A)
5
Introduction
How to Use this Manual
This manual is a reference for the use of Neural Analytics’ NeuralBot™ System for the Lucid™
M1 Transcranial Doppler ultrasound system. The manual is intended to help the user operate
the system. To avoid any operating errors please read these instructions carefully before using
the device.
Indications for Use
The NeuralBot™when used with the Lucid™M1 System is a medical ultrasound device which
assists the user in the setup and acquisition of cerebral blood flow velocity via the patient’s
temporal windows. It is intended for use as an adjunct to standard clinical practices for
measuring and displaying cerebral blood flow velocity and the occurrence of transient emboli
within the blood stream.
The NeuralBot™is intended to be used by healthcare professionals qualified by training in its
safe and effective use. The device is not intended to replace other means of evaluating vital
patient physiological processes, is not intended to be used in fetal applications and is not
intended to be used inside the sterile field.
Contraindications
The NeuralBot™System is not intended to be used in persons younger than 18 years of age.
The NeuralBot™System is not intended to be used in fetal applications.
The NeuralBot™System is not intended to be used inside the sterile field.
Intended Use Environment
The NeuralBot™System is intended to be used on patients in the supine or reclined position up
to 45 degrees on a bed or a gurney in a clinical setting. The device is NOT intended to be used
simultaneously with head imaging equipment such as MRI and CT.
Compatibility
The NeuralBot™System is compatible with the following:
1. Lucid™M1 System, Software revision 1.3.4 or higher.
2. NeuralBot™Procedural Set (NA-DSP2).
NeuralBot™ System User Manual (60-00119, Rev. A)
6
Conventions used in this Manual
Throughout this manual, the following conventions are utilized:
Caution indicates a potentially hazardous situation that may result in minor or moderate
injury to the patient or user or damage to the system.
Precaution indicates an action that a patient or user can initiate to prevent injury to a
patient or user or damage to the system.
WARNING indicates a significant problem that may lead to serious injury or death to the
patient or user or may damage the NeuralBot™system.

NeuralBot™ System User Manual (60-00119, Rev. A)
7
Symbols
Symbol
Description
Symbol
Description
Caution or Precaution
WARNING
Serial number
Lot Number
Direct current
Fragile, handle with
care
Alternating current
Keep dry
Equipotential ground
Temperature limits
(See Technical
Information)
Humidity limits (See
Technical Information)
Atmospheric pressure
limits (See Technical
Information)
Manufacturer
On/Off Push button or
switch
DC power connection
Catalog number
Consult instructions for
use
Protective Earth
Terminals
Refer to instruction
manual
For indoor use only
USB2.0 or USB 3.0 that
is backwards-compatible
to USB 2.0
Cart Tipping Hazard
B-type Applied Parts
Prescription Only
CE Marking
Separate collection for
electrical and
electronic equipment
(WEEE)
European authorized
representative

NeuralBot™ System User Manual (60-00119, Rev. A)
8
Product Description
Overview of the NeuralBot™System
The NeuralBot™System is a robotic probe positioning device that is paired with the Lucid™M1
System. It is designed to assist in the acquisition of cerebral blood flow velocity data from the
arteries and veins of the head via the patient’s temporal windows. The NeuralBot™ System
uses two reusable, non-sterile 2-MHz probes that are controlled by robotic actuators. The
NeuralBot™System is a cart mounted system and contains the following components.
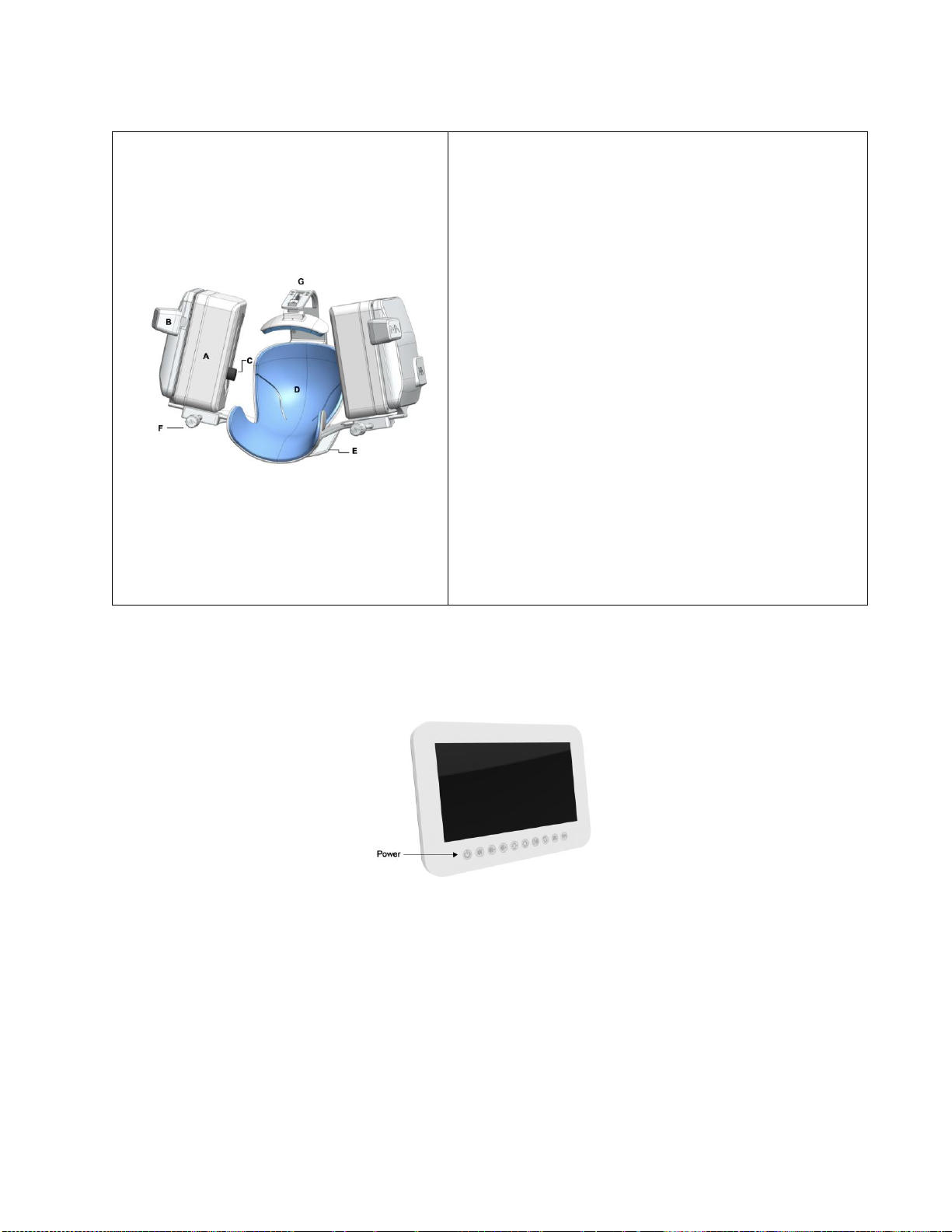
Figure 1: NeuralBot™System Components
A. Patient Headmount Unit (PHU): The unit that
consists of the head cradle, headmount base, the
left and right probe pods and the head stabilizer.
B. Lucid™M1 System: The TCD ultrasound system
(for reference only).
C. Accessory Control Unit (ACU): The touchscreen
computer in which the NeuralBot™system is
operated.
D. Robotic Control Unit (RCU): The control unit that
contains the electronics and embedded software.
E. Cart: Medical grade cart with VESA mounts and a
flat work surface and storage shelves.
F. Storage Bin: Container attached to the back of
the cart that can be used to store the headmount
base and head stabilizer.
NeuralBot™ System User Manual (60-00119, Rev. A)
9
Figure 2: Patient Headmount Unit
A. Probe Pod (Left and Right): Detachable pods
that contain the TCD probes and robotic
actuators.
B. Registration Camera (Left and Right): Camera
that registers the dots applied to the patient’s
face to determine the search range area.
C. Probes (Left and Right): Probes used to gather
TCD signals.
D. Head Cradle: The support structure in which the
patient’s head is placed.
E. Headmount Base: The support structure of the
patient headmount unit.
F. Headmount Screws (Left and Right): Screws
used to tighten or loosen the probe pods.
G. Head Stabilizer: A detachable brace on the head
cradle. Used to stabilize and hold the patient’s
head in place. The position of the stabilizer and
firmness applied to the head are adjustable.
Figure 3: Accessory Control Unit (ACU)

NeuralBot™ System User Manual (60-00119, Rev. A)
10
Figure 4: (Top) Frontal view of Robotic Control Unit (RCU); (Bottom) Rear of the RCU showing
locations of (A) Probe Pod receptacles –left and right sides; (B) Power Switch
Figure 5: Probe Pod Storage Tray is used to store the Probe Pods when not in use
Package Contents
The NeuralBot™System is shipped in four separate packages
1. Robotic control unit (NA-RCU1) and Patient headmount unit (NA-PHU1)
2. Accessory Control Unit (NA-ACU1)
3. System cart configuration (NA-ACC10)
4. The NeuralBot™Procedural Set which includes:
•4 registration dots
•1 padding for the head cradle
•1 padding for the forehead mount
•2 probe pod covers
oThe NeuralBot™Procedural Set is shipped in packages of 10 (NA-
DSP2PK).
NeuralBot™ System User Manual (60-00119, Rev. A)
11
Cautions and Warnings
WARNING
US Federal Law restricts this device to use by or on the order of a physician.
Read User Manual before using system.
Use only approved accessories with this device. See Compatibility section for list of approved
accessories.
Minimize ultrasound exposure and use the principle of As Low As Reasonably Achievable
(ALARA).
Do not place the NeuralBot™system over other device electrodes, sensors or cables.
Caution should be used when placing NeuralBot™system over nasal cannula.
Do not place the NeuralBot™system over open wounds or abrasions.
Do not push the probe pods with excessive pressure against the patient’s head.
Do not insert objects, gel or liquids into the probe pod cavities.
Patient contact materials are only intended for limited duration (less than 24 hours) skin contact.
Do not use device in a nitrous oxide, oxygen-rich or explosive atmosphere or in the presence of
explosive anesthetics.
Cardiac rhythm disturbances during perfusion studies using gas ultrasound contrast agents
have been observed in the diagnostic range.
Inspect the system prior to use. Please contact Neural Analytics if any damage or deterioration
is observed for the system, including the transducer probes, cart and cables.
Do not use transducer probes if the transducer probe face, body or cables are damaged as this
may cause patient discomfort when used.
Do not use if any part of the system is dropped as function may be compromised.
Do not modify the system. Modifications may degrade the measurements, impact the service
life of the product or cause hazardous situations such as electric shock.
To avoid risk of damage to the device:
Connect only to the correct rated mains outlet.
Always use the provided probe pod covers prior to operating the system. Do not
perform scans without using the covers.
Do not operate in outdoor lighting conditions.
Do not use alcohol for cleaning and disinfection as it may damage the probe transducer;
wipe the probe transducer with a clean damp cloth immediately after use.
To avoid risk of electric shock:
The equipment must only be connected to supply mains with protective earth.
Keep liquids and gels away from the NeuralBot™openings and electrical connections.
Place covers on the left and right probe pods prior to use.
Do not open the device enclosure.

NeuralBot™ System User Manual (60-00119, Rev. A)
12
Disconnect device from AC Adapter prior to cleaning.
Inspect the AC Adapter and AC cord set for damage prior to use.
Connect to "Hospital Only" or "Hospital Grade" or equivalent receptacles.
Do not submerge the device in liquid. This will damage the device.
When disconnecting any electrical connection from the device, pull from the plug and not the
cable.
Check the audio volume to avoid hearing loss or damage especially when using headphones.
Store cables away from the ground to avoid tripping during use or transportation.
Do not block or cover vents. This may cause the device to overheat during use.
This device contains both a lithium-ion battery and a lithium polymer battery. It must be
disposed of properly. Follow your clinic or hospital disposal or recycling procedures.
This device meets EMI/EMC standards.
However, this device may cause radio interference or may disrupt the operation of
nearby equipment. If necessary, move the device to a difference location or re-orientate.
Electromagnetic fields from other non-compliant devices may cause degradation of the
device display. If necessary, move the source of interference to a different location.
This device contains a multiple socket-outlet (MSO). Connecting electrical equipment to the
MSO effectively leads to creating a medical electrical system and can result in a reduced level
of safety. Do not connect any other electrical equipment to the MSO.
Additionally, do not connect an MSO or extension cord to the system. Connect to “Hospital
Only” or “Hospital Grade” or equivalent receptacles.
When not using the device, store in a location to minimize accidental damage to the equipment.
When shipping the device, use only Neural Analytics approved shipping package.
Shipping the device in an unapproved container may damage the equipment. Call Neural
Analytics Customer Service for assistance in shipping a Neural Analytics device.
Use of this device in the presence of an ESU (electrosurgical generator) or electrocautery may
cause distortion in the display.
Read and follow all cleaning and disinfection instructions. Reference the Equipment Care and
Maintenance section of this manual for additional cleaning instructions.
To avoid risk of tipping the cart:
Do not apply a force greater than 20 lbs. at 1.5m or above to the cart.
Do not lean on the cart.
Prior to moving the system, lower the cart so that the keyboard shelf is just above the
probe pods.
Make sure wheels are unlocked before attempting to move the cart.
Use caution and pull the cart over bumps or uneven surfaces. Do not push the cart
over thresholds, bumps or uneven surfaces.
Lock all four cart wheels after moving.

NeuralBot™ System User Manual (60-00119, Rev. A)
13
Clinical Safety
There are no confirmed biological effects on patients or equipment operators caused by
exposure from existing ultrasound equipment in the market.
Ultrasound Safety
Biological interaction and Ultrasound Bio-effects
As ultrasound energy is propagating into the body, the soft tissue absorbs or scatters some of
the energy. The scattering of ultrasound energy may cause reflections or echoes on the
spectrogram. Absorption of ultrasound energy may cause tissue damage by one or a
combination of two methods: a mechanical mechanism which may include cavitation and a
thermal mechanism that causes heating. Following As Low as Reasonably Achievable (ALARA)
mitigates the possibility of any of these mechanisms occurring.
ALARA
When using NeuralBot™, the principles of ALARA should always be followed. ALARA is the
acronym for As Low As Reasonably Achievable. This principle simply states that the operator
should keep the ultrasound output levels and exposure times as low as possible while still
obtaining the desired Cerebral Blood Flow Velocity information.
Managing Ultrasound Exposure
Ultrasound energy may cause tissue damage due to mechanical or thermal mechanisms. To
assist users, thermal and mechanical indexes are modeled to represent relative measure of
potential tissue damage. For tissue damage due to heating, the thermal index is displayed to the
user. For more detail related to the mechanical index, see the Lucid™M1 User Manual.
Personnel must be trained by Neural Analytics on how to use the NeuralBot™system. The user
should be familiar with the document “Medical Ultrasound Safety” from the American Institute of
Ultrasound in Medicine (AIUM). This document may be ordered from AIUM (www.aium.org).
Review the Neural Analytics Lucid™M1 System User Manual section on Operating Controls
prior to using this equipment.

NeuralBot™ System User Manual (60-00119, Rev. A)
14
Operating NeuralBot™
Overview of the NeuralBot™procedure
The following steps provide a general overview of the NeuralBot™System procedure. Refer to
the following sections for more information about each of these steps.
1. System Pre-Checks
2. Patient Setup
3. Perform Exam
4. Post Exam procedure
5. Quick Release procedure
6. System power down
7. Clean the system
System Pre-Checks
This section outlines the steps to ensure the system is ready for use prior to performing an
exam.
Connections and Power
1. System Cart
a. Move the cart to the intended area and connect the power to a wall outlet.
b. Lock the cart’s wheels.
c. If a wall outlet is not available, the system will operate on battery power for up to
30 minutes. The system will notify the user when 15% power is remaining.
2. Accessory Control Unit (ACU):
a. Power ON by pressing and holding the power button. Log in to the Accessory
Control unit.
•Factory default password: password
3. Robotic Control Unit (RCU):
a. Confirm the probe pod connector cables (x2) are connected to the two
corresponding (Left/Right) RCU connectors.
•Position the system to limit bending or straining on cables and
connections.
b. Power ON the RCU by pressing the power switch on the RCU.
•Confirm the RCU Power indicator light is green. Confirm the Headset
Power indicator light is green.
4. Patient Headmount Unit (PHU)
a. Set the headmount base on a flat surface.
b. Carefully remove the left and right probe pods from the cart tray and attach
to the corresponding rails on the headmount base.
c. Confirm that the left and right probe pod connector cables are connected to the
corresponding left and right probe pod connectors.

NeuralBot™ System User Manual (60-00119, Rev. A)
15
5. Lucid™M1 System:
a. Connect the left and right TCD cables on the probe pods to the left (green) and right
(orange) connectors on the Lucid™M1.
•Position the system to limit bending or straining on cables and connections.
b. Power ON by pressing and holding the power button.
c. Log in to the Lucid™M1 System (refer to Lucid™M1 System for additional
setup details).

NeuralBot™ System User Manual (60-00119, Rev. A)
16
Configure and Initialize System
On the NeuralBot™ACU:
1. On the “Home” screen, press the
flashing blue arrow on the
bottom right to proceed.
2. The “Setup” screen displays system
connection statuses. The left and
right RCU status will display
“Connected”.
3. Click on the flashing Initialize
button.
•Confirm the probe pods are not
obstructed prior to initialization.
•The system will perform its self-
test process. Upon completion,
the system will display
“Initialized” on the left and right
RCU statuses.

NeuralBot™ System User Manual (60-00119, Rev. A)
17
Patient Setup
1. On Lucid™M1, create or
select a patient. See Lucid™
M1 User Manual for more
detail.
2. Select a Lucid™M1 exam
type on the Exam
Procedure screen. See
Lucid™M1 User Manual for
more detail.
•The left and right Lucid™
status will display
“Connected”on the ACU.
•NOTE: Before and/or during
signal search, do not change
Lucid™M1 parameters.
Changing parameters may
affect signal search.
•For unilateral exams, disable the channel not in use by pressing the button for that
channel.
3. Make sure the patient is lying flat or in a reclined position no greater than 45 degrees.
4. Open the NeuralBot™Procedural Set which includes the following non-sterile, single use
items:
(x2) Comfort Paddings
(x2) Probe Pod covers
(x4) Registration dots
5. Locate the two comfort paddings.
•Firmly attach the larger comfort
padding to the head cradle after
removing the backing layer. Make
sure there are no air pockets
between the comfort padding and
the head cradle.
•Firmly attach the smaller
comfort padding to the head
stabilizer after removing the
backing layer.

NeuralBot™ System User Manual (60-00119, Rev. A)
18
6. Locate the probe pod covers and
carefully cover each probe pod.
Insert the probes and registration
cameras through the corresponding
cut outs.
•Probe: Center Cut Out
•Camera: Corner Cut Out
Caution: Use of the NeuralBot™
System without probe pod covers may
result in harm to user or patient, or
equipment damage.
7. On each side of the patient’s head
apply one registration dot to the
mid ear and one to the outside
corner of the eye - a total of four
registration dots. Make sure they are
firmly in place.
8. Apply ultrasound gel between the
registration dots on each side of
the patient’s temporal region.
Make sure not to get gel on the
registration dots.

NeuralBot™ System User Manual (60-00119, Rev. A)
19
9. Position the left and right probe pods
symmetrically on the PHU railings.
10. Carefully rest the patient’s head
into the PHU. Make sure that the
patient’s head is symmetric within
the PHU and that the top of the
patient’s head is in contact with the
top of the head cradle.
•For patients with long hair,
make sure that their hair is
untied and worn so that it does
not obstruct their temporal
region.
11. Attach the head stabilizer and
firmly secure on patient’s forehead.
Position the brace above the
patient’s eyebrow.

NeuralBot™ System User Manual (60-00119, Rev. A)
20
12. Press the flashing next arrow on the
bottom right to advance to the
“Registration”screen.
Table of contents