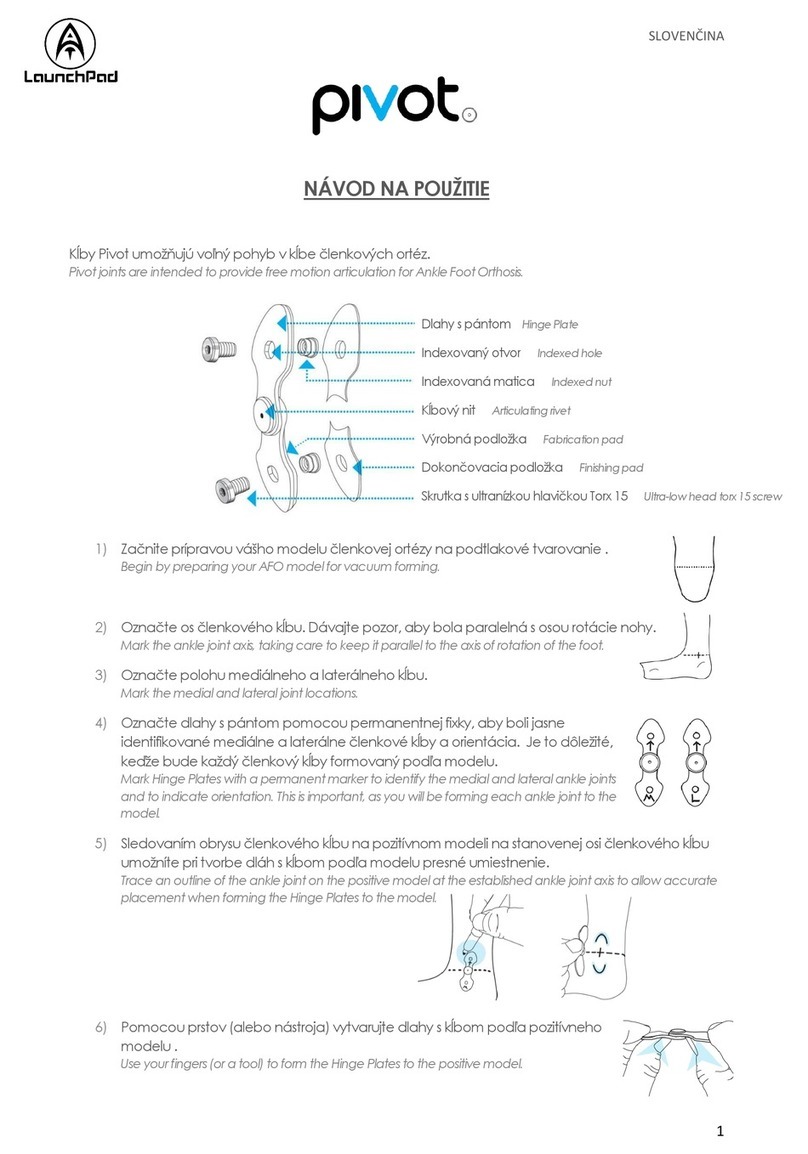
Neurosign 100 User manual

August 2011
Neurosign 100
Operating Manual
NOP01-EN
Revision 02

Neurosign 100
Operating Manual
© The Magstim Company Limited i NOP01-EN-02
TABLE OF CONTENTS
Table of Contents ................................................................................................................. i
Guarantee........................................................................................................................... ii
Section 1 Introduction....................................................................................................... 1
1.1 Indications for Use................................................................................................... 1
1.2 Contraindications..................................................................................................... 1
1.3 Devices Covered..................................................................................................... 1
Section 2 Warnings and Precautions ................................................................................. 2
Section 3 Product Descriptions.......................................................................................... 4
3.1 Device Description................................................................................................... 4
3.2 Front Panel ............................................................................................................. 4
3.3 Rear Panel.............................................................................................................. 6
3.4 Pre-amplifier Pod..................................................................................................... 7
3.5 Stimulator Pod......................................................................................................... 7
3.6 Mute Sensor............................................................................................................ 8
3.7 Accessories ............................................................................................................ 8
Section 4 Operating Instructions........................................................................................ 9
4.1 Preparation ............................................................................................................. 9
4.2 Connection.............................................................................................................. 9
4.3 Operation.............................................................................................................. 10
4.4 Interpreting Neurosign 100 Sounds......................................................................... 10
4.5 Troubleshooting..................................................................................................... 12
Section 5 Maintenance ................................................................................................... 13
5.1 User Maintenance and Calibration.......................................................................... 13
5.2 Voltage Selection and Fuse Rating......................................................................... 13
5.3 Cleaning and Disinfecting....................................................................................... 13
5.4 Servicing............................................................................................................... 13
5.5 Device Lifetime...................................................................................................... 13
5.6 Disposal................................................................................................................ 13
Section 6 Specifications.................................................................................................. 14
6.1 Safety Specifications.............................................................................................. 14
6.2Technical Specifications......................................................................................... 15
6.3 Environmental Conditions ...................................................................................... 16
6.4 Packing Instructions............................................................................................... 16
Section 7 Contact Details................................................................................................ 17
7.1 Product Enquiries.................................................................................................. 17
7.2 Servicing Enquiries................................................................................................ 17
7.3 Sales Enquiries ..................................................................................................... 17
APPENDIX A –STIMULATION CURRENTS.................................................................... 18
APPENDIX B –ELECTRODE PLACEMENT DIAGRAM ................................................... 19
APPENDIX C –MANUFACTURER’S EMC DECLARATIONS ........................................... 21

Neurosign 100
Operating Manual
© The Magstim Company Limited ii NOP01-EN-02
GUARANTEE
Equipment manufactured by The Magstim Company Limited is fully guaranteed, covering
both materials and workmanship, for a period of one year from the date of shipment. The
Magstim Company Limited reserves the right to perform guarantee services in its factory, at
an authorised repair station, or at the customer’s installation.
Reusable stimulating probes are guaranteed, covering both materials and workmanship, for
a period of 3 months from the date of shipment.
Reusable needle electrodes are guaranteed, covering both materials and workmanship, for
a period of 1 month from the date of shipment.
Single use needle electrodes and stimulating probes are guaranteed to be free of defect
upon use. Sterilisation is guaranteed subject to the packaging being undamaged and the
product being within the serviceable life as stated by the sterilisation date shown on the
label.
Any obligations which The Magstim Company Limited has under this guarantee are limited
to repairs or, should the company so choose, replacement of any defective parts of the
equipment, except batteries, without charge provided that the said defects occur during
normal service.
Claims for damages during shipment must be filed promptly with the transportation
company. All correspondence concerning the equipment must specify the model name
and/or number as well as the serial number exactly as they appear on the invoice for the
equipment.
Improper use, mishandling, tampering with, or operation of the equipment without following
specific operating instructions will void this guarantee and release The Magstim Company
Limited from any further guarantee obligations.
The Magstim Company Limited will only accept responsibility for effects on safety, reliability
and performance of the equipment if:
modifications or repairs are carried out by persons authorised by The Magstim
Company Limited;
the electrical installation of the relevant room complies with local regulations; and
the equipment is used in accordance with the instructions for use.

Neurosign 100
Operating Manual
© The Magstim Company Limited 1 NOP01-EN-02
SECTION 1 INTRODUCTION
1.1 Indications for Use
The Neurosign 100 Nerve Monitor has been designed to meet the needs of operating theatre staff by
continuously monitoring motor neural pathways which are at risk during surgical procedures.
The instrument gives an audible interpretation of muscle activity, which is sensed by either needle
electrodes placed into the relevant muscles controlled by those nerves to be monitored or by a
Laryngeal Electrode placed around an endotracheal tube prior to intubation.
A number of outputs are available on the rear of the Neurosign 100 Unit enabling connection to other
equipment for analysis either during or post surgery.
1.2 Contraindications
The Neurosign 100 is contraindicated for use with paralyzing anaesthetic agents that will significantly
reduce, if not completely eliminate, EMG responses to direct or passive nerve stimulation.
1.3 Devices Covered
This document is applicable to the following devices.
Neurosign 100 Nerve Monitor (P/N: 9883-01)
Neurosign 100 Pre-amplifier Pod (P/N: 1719-00)
Neurosign Stimulator Probe Pod (P/N: 9967-00)
Mute Sensor (P/N: 1020-00)
Note: Please also consult any labelling and information accompanying stimulating probes and
electrodes for safety and use information regarding these devices.
Always consult labelling and any instructions for use accompanying stimulating probes and electrodes
for use with the Neurosign 100.

Neurosign 100
Operating Manual
© The Magstim Company Limited 2 NOP01-EN-02
SECTION 2 WARNINGS AND PRECAUTIONS
Attention: consult accompanying documentation before using the device(s).
Type BF Applied Part: Refer to Section 6.1 for further information.
High Voltages: High voltages are present in the Neurosign 100 during operation. Do not remove
covers. Refer servicing to qualified personnel. If there is any sign of external damage or if any parts
are damp or wet, they must not be used.
Flammable Anaesthetics: The Neurosign 100 and accessories must not be used in an explosive
atmosphere or in the presence of flammable anaesthetics.
Neurosign Users: The Neurosign 100 is designed for use during surgical procedures and must only
be used by qualified personnel. This instrument is designed as an aid to surgeons in the
identification and monitoring of motor nerves at risk during surgery. It is not a replacement for
experience in surgical decision-making. USA: RX Only
Neuromuscular Block: Muscle contractions are only possible if limited or no neuromuscular block
is used. The Neurosign 100 may not respond to spontaneous stimulation in instances where the
patient is paralysed. The train of four test should not be used as proof that any blockade has
dissipated; the Neurosign 100 can detect EMG well below the threshold of visible muscle movement.
It is recommended that a suitable anaesthetic protocol is implemented so as not to impede muscle
movement.
Electrical Safety: At no point should users or third parties be allowed to come into contact with the
patient and the output connectors on the rear of the Neurosign 100 unit simultaneously.
Rear Panel Outputs: Only equipment that meets the relevant IEC standards and is configured in
compliance with IEC 60601-1-1, should be connected to the Neurosign 100.
Mute Sensor: When not in use, disconnect the Mute Sensor from the Neurosign 100 unit. Spurious
muting may occur if the Mute Sensor is left connected while not being used.
Current Control: Currents in excess of 1mA should not be used to directly stimulate nerves during
surgery involving cranial nerves.
Stimulating Probes: Stimulating probes should not be left standing in saline or allowed to come into
contact with other electrical devices when being used.
Disconnection: Parts of the Neurosign 100 system should not be disconnected by pulling on the
connection cable as damage may occur.

Neurosign 100
Operating Manual
© The Magstim Company Limited 3 NOP01-EN-02
Ventilation: Care should be taken so that ventilation holes on the Neurosign 100 unit are not
obstructed.
Damage: If any cracks or damage are found on the Neurosign 100 system, it must not be used and
should be returned to The Magstim Company Limited for servicing.
Electrosurgical Equipment: The Neurosign 100 should only be used with surgical equipment which
has an automatic cut-out feature and measures return pad impedance. It is suggested that bipolar
diathermy should be used instead of monopolar diathermy as bipolar diathermy uses its own active
return electrode.
Stray RF: In order to reduce any possible induced current in the needle electrode leads from stray
RF emitted by the electrosurgical unit, the electrode leads should be kept as far as possible from the
unit.
Cables from the electrosurgical unit, both active and return, should also be kept as far as possible
from the electrode leads, especially whilst monopolar diathermy is being used.
Magnetic Fields: The Neurosign 100 must not be used in the vicinity of objects that are sensitive to
magnetic fields.

Neurosign 100
Operating Manual
© The Magstim Company Limited 4 NOP01-EN-02
SECTION 3PRODUCT DESCRIPTIONS
3.1 Device Description
The Neurosign 100 provides an audio and visual representation of small voltages detected within a
group of muscles.
The voltages are detected by the sensing electrodes which are placed adjacent to or into the muscle
group. Pulses or clicks can be heard when stimulating tissue using a stimulation probe indicating
nervous tissue is being stimulated.
3.2 Front Panel
The front panel, shown below, allows control of the N100 unit and provides the connection for the
stimulator pod.
3.2.1 Frequency (1)
The frequency switch has 2 positions labelled 30Hz and 3Hz and controls the frequency at which the
stimulator current is applied through the stimulating probe. Typically this should be set to 30Hz. In
situations where muscular block has to be used, this should be set to 3Hz.
3.2.2 Probe (2)
The Stimulator Probe Pod should be connected to the probe socket if required during the procedure.
3.2.3 Current (3)
The current control varies the amount of current delivered to the Stimulator Probe Pod. Turning the
control clockwise increases the current. Turning anticlockwise decreases the current.
Most of the travel of the control is on the lower current levels. Current is delivered as a 200µs pulse at
the set frequency. Please see Appendix A for suggested stimulation current settings.
1
2
4
3
5
9
7
8
6

Neurosign 100
Operating Manual
© The Magstim Company Limited 5 NOP01-EN-02
3.2.4 Current Confirm (4)
Current confirm will illuminate as an indication that the correct current has passed through the
stimulating probe. If the Frequency is set to 3Hz, it may take 2 or 3 seconds for the Current Confirm to
illuminate.
If the current confirm is permanently lit or does not light as expected, this could be an indication that a
probe is faulty. Please refer to Section 4 Operating Instructions.
3.2.5 Channel 1 and Channel 2 (5)
Each channel has a switch to turn it on or off. When the channel is off the on/off indicator will be
orange. When the channel is on the on/off indicator will be green.
3.2.6 Electrode Warning (6)
Each channel has an Electrode Warning indicator and this will illuminate if a level of artefact or
unwanted voltages are detected by the Neurosign 100 unit continuously for a period of 20 seconds. A
short beep will repeat every 8 seconds until the problem is cleared.
3.2.7 Bar Graph (7)
Each channel has a bar graph indicator which will illuminate showing the level of EMG activity
detected by the Neurosign 100 unit. When EMG is detected, the bar graph rises to the detected level.
When EMG ceases, the bar graph falls, however, the top segment of the bar graph will remain
illuminated for several seconds to allow the EMG level to be determined by the user. The Bar Graph
is independent to the volume and is not altered by changes to the volume level.
3.2.8 Volume (8)
The volume control allows the volume of the internal speaker to be altered. Turning the control
clockwise increases the volume. Turning anticlockwise decreases the volume. Please note: The
volume cannot be set to zero. This is an intentional safety feature.
3.2.9 Channel Ident (9)
When switched on, channel ident introduces a different texture to the sound of each channel heard
through the speaker and can be used to differentiate between the two channels when they are on
simultaneously.

Neurosign 100
Operating Manual
© The Magstim Company Limited 6 NOP01-EN-02
3.3 Rear Panel
The rear panel, shown below, provides connections for power, headphones, pre-amplifier pod, mute
sensor and outputs for connection to external equipment.
3.3.1 Power (1)
The Power switch allows the power supplying the unit to be switched on (also labelled I) or off (also
labelled O).
3.3.2 Mains Connector (2)
The standard IEC mains lead should be connected to the unit prior to the unit being switched on.
3.3.3 EMG Output (3)
The EMG Outputs provide the ability to connect to, and view or record, EMG waveforms or signals
externally to the Neurosign 100. These outputs are taken following the 10kHz filters within the
Neurosign 100 unit, but before any signal processing occurs. The signal amplifiers have a gain of
500 and an output in the range of 15mV to 10V peak-to-peak.
3.3.4 Trigger Output (4)
The Trigger Output provides the ability to connect to external devices to trigger or synchronise with
the Neurosign 100 Unit. The trigger signal output is at the same frequency as that set on the front
panel: either 3Hz or 30Hz.
3.3.5 Mute Sensor (5)
The Mute Sensor socket allows connection of a mute sensor to the Neurosign 100 unit.
3.3.6 Stimulation Current Record (6)
The Stimulation Current Record output allows external connection to view or record the stimulation
current applied to the patient. It provides a voltage output proportional to the stimulation current
being delivered and is only present when Current Confirm is illuminated.
1
2
4
9
5
8
3
6
7

Neurosign 100
Operating Manual
© The Magstim Company Limited 7 NOP01-EN-02
3.3.7 Headphones Volume (7)
The Headphone Volume is controlled by a thumbwheel knob. Rotating the thumbwheel upwards
increases the volume. Downwards decreases the volume.
3.3.8 Headphones Socket (8)
The headphone socket allows connection of headphones with a 3.5mm jack plug.
3.3.9 Pre-amp Pod Input (9)
The Neurosign 100 pre-amplifier pod should be connected to this socket.
3.4 Pre-amplifier Pod
The Pre-amplifier Pod provides connections for electrodes. The Pre-amplifier Pod connects to the
Pre-amp Pod connection on the rear panel.
3.5 Stimulator Pod
The Stimulator Pod provides connections for stimulating probes. The Stimulator Pod connects to the
Probe connection on the front panel.

Neurosign 100
Operating Manual
© The Magstim Company Limited 8 NOP01-EN-02
3.6 Mute Sensor
The Mute Sensor enables the sound artefact from monopolar and/or bipolar electrocautery to be
muted and connects to the Mute Sensor socket on the rear panel. Bar graphs on the front of the
Neurosign 100 and EMG outputs on the rear of the Neurosign 100 will show a high level of artefact
during electrocautery.
3.7 Accessories
A range of stimulating probes and electrodes are available for use with the Neurosign range of
products. For more details, please contact the Sales department. Contact details are shown in section
7.
Please note: The use of the correct accessories is essential to the functioning of the Neurosign 100.
The Magstim Company Limited cannot guarantee the instrument’s performance unless accessories
used are obtained from The Magstim Company Limited. Only Neurosign accessories purchased from
The Magstim Company Limited should be used with the Neurosign 100 system.
.............…….

Neurosign 100
Operating Manual
© The Magstim Company Limited 9 NOP01-EN-02
SECTION 4OPERATING INSTRUCTIONS
Note: Please ensure you are familiar with all sections of this operating manual prior to following these
operating instructions.
4.1 Preparation
1. At the start of each session the operator must check the Neurosign 100, Pre-amplifier Pod and
Stimulator Pod for any signs of external damage and to identify any cleaning required. If any
cracks are visible in the housing, or there is damage to any of the cables, the items must not be
used and should be returned to The Magstim Company Limited for servicing and repair. If
cleaning is required, please follow the instructions in Section 5 of this Operating Manual.
2. Ensure that any electrodes or stimulating probes to be used are sterile. Re-usable electrodes and
probes should be sterilised prior to use. Single use electrodes and probes should be supplied
sterile.
3. Ensure that the Neurosign 100 unit is switched off on the rear panel and that Channel 1, Channel
2 and Channel Ident are switched off on the front panel.
4.2 Connection
1. Insert the electrode(s) into the patient and connect them to the pre-amplifier pod as directed by
the electrode(s) accompanying documentation. Information on electrode placement can be found
in Appendix B. Additional information may be available from The Magstim Company Ltd. if
necessary. Please refer to Contact Details in Section 7.
2. Attach the Pre-amplifier Pod to the patient or bed and plug the cable into the pre-amp pod input
on the rear panel of the Neurosign 100.
3. If a stimulating probe is to be used, connect the stimulating probe to the Stimulator Pod as
directed by the probe(s) accompanying documentation. Attach the Stimulator Pod to the Probe
socket on the front of the Neurosign 100 unit. If probes are connected to both sockets, it is
important to realise that both probes will be “live” when stimulation is active. Attach the Stimulator
Pod to the patient or bed.
Please note: The Pre-amplifier Pod and Stimulator Pod are not sterile and should be positioned
in the non-sterile area. It is advised that the Pre-amplifier Pod is positioned at the top of the
operating table near the patients head, with the Stimulator Pod at the bottom of the table near the
patient’s feet.
4. The Neurosign 100 can reject high level input signals such as those caused by using
electrocautery equipment, however, if muting of the Neurosign 100 sound output is required
during electrocautery, connect the Mute Sensor to the Mute Sensor socket on the rear of the
Neurosign 100 and clip the sensor to the electrocautery cable near to the diathermy machine.
Please note: If muting is not required, the Mute Sensor should not be connected. Spurious
muting may occur is the Mute Sensor is connected when not required.
5. Headphones, connections to external equipment and other required items should be connected
as necessary.
6. Raise the front of the Neurosign 100 by altering the handle position to allow low level sounds to
be heard.

Neurosign 100
Operating Manual
© The Magstim Company Limited 10 NOP01-EN-02
4.3 Operation
1. Connect the power lead and switch the Neurosign 100 unit on. Current Confirm should not be
illuminated. Channel 1 and Channel 2 indicators should be orange.
2. Turn on Channel 1 and Channel 2 as required. The orange indicator should change to green
when the channel is switched on.
It is normal for segments of the bar graph to flicker for the first few minutes following needle
insertion but no segments should remain lit. Permanently lit segments may indicate incorrect
electrode placement and electrodes should be removed and reconnected to the patient to rectify
this. Electrode placement should be checked before the patient is draped.
The probe can also be checked at this point by shorting the tips together using a damp cloth.
When shorted the Current Confirm LED should illuminate.
3. Set the volume to the desired level and, if required, switch on Channel Ident to allow
differentiation between the audio of Channel 1 and Channel 2.
4. Set the Frequency switch to the desired setting. This is usually set to 30Hz except in cases where
the patient has to be paralyzed for the whole procedure when this should be set to 3Hz.
Responses to stimulation will be slow at 3Hz.
5. Set the Stimulator Current to the lowest level appropriate for the procedure. At this point, there
should be no sound output or bar graph activity other than that associated with spontaneous firing
of the nerve.
6. Check the area for nerve fibres. When the Stimulating Probe comes into contact with a neural
pathway, the instrument will register a response dependant upon the level of stimulation. If there
is either a weak or no reaction from the unit, the Stimulator Current may be increased.
7. Set an appropriate Stimulator Current according to the guidance in Appendix A.
8. On completion of the procedure, Section 4.2 should be repeated in reverse to disconnect the
Neurosign 100.
4.4 Interpreting Neurosign 100 Sounds
There are four basic sounds from the instrument when used correctly:
1. A long series of pulses lasting as long as the nerve is stimulated is due to direct electrical
stimulation. The volume of the signal will vary according to the current being passed, the amount
of neuromuscular block (if any) used, and the integrity of the nerve.
2. A burst of clicks is caused by direct manipulation of the nerve. This is caused by a brief
mechanical contact caused by the surgeon working close to the nerve and ends as the contact is
removed.
3. A regular, repetitive ‘train’ of clicks lasting several seconds or even minutes. This ‘train’ of pulses
may be caused by mechanical manipulation of the nerve, by compressing or stretching it, or by a
change in temperature, as when irrigating the area with saline or heating the area by diathermy or
with a laser. Unless the train of pulses is caused by irrigation, it may be advisable to allow the
nerve to recover before continuing with the procedure.
4. An absence of sound could be caused because the instrument has not detected any voltage in
the muscle and the muscle must contract in order for a sound to be heard. The muscle may not
contract for a variety of reasons; the nerve may not be stimulated, the nerve may be damaged or

Neurosign 100
Operating Manual
© The Magstim Company Limited 11 NOP01-EN-02
transected, or any neuromuscular block may be too deep. Silence may also be a sign that the
surgery is not irritating the nerve.

Neurosign 100
Operating Manual
© The Magstim Company Limited 12 NOP01-EN-02
4.5 Troubleshooting
Below are some potential cures to problems you may experience.
Problem
Potential Cure
No indicators light when power is
switched applied
Check Power Switch is set to 1: check power socket; check voltage
selector; check unit fuses
Background theatre noise obscures
Neurosign 100 information
Increase volume or use headphones
Loud noise when using electrocautery
Attach the mute sensor and clip it to the electrocautery cable –
bipolar diathermy should not affect the Neurosign 100, but this can
also be muted if necessary
No response at start of operation
This is normal! It may take a long time to reach the nerve or tissue to
which the nerve is attached; if in doubt, use the stimulator set at a
high current (2-3mA) to check the area –a faint clicking may be
heard indicating the nerve lies deep to the probe
Little or no mechanically evoked
responses
If any neuromuscular block has been used, only direct electrical
stimulation is likely to be heard –the train of four test is not a
guarantee that the neuromuscular junction is free of blockade
Constant clicking noise and bar graph
display with no stimulation; Electrode
Warning indicator on and beep
sounding
Part of the bar graph has been lit for more than 20 seconds. The
electrodes may be poorly placed or faulty, giving rise to artefact.
Check if possible before the patient is draped
No response when using Stimulator
a) If a neuromuscular block has been used, set Frequency to 3Hz
b) If neuromuscular block is deep, a response is unlikely
c) Is the Current Confirm indicator lighting?
d) Increase current setting up to a maximum of 1mA
e) Did the patient have a normal nerve pre-operatively? (!!)
Current Confirm indicator stays lit after
Stimulation is finished
Check for liquid material between probe tips and clean as
necessary. Concentric probes may cause the indicator to stay lit for
several seconds because of the proximity of the electrodes
Bipolar probe is too big under the
microscope and penetrates too far
through tissue
The concentric probes have a diameter of 1mm and also have very
much reduced current spread, making these probes suitable for use
under the microscope and, for example, differentiating between VIIth
and VIIIth cranial nerves . Alternatively, try the precision bipolar
probe
Difficulty in differentiating between the
two channels
Set INDENT feature to ON, and ensure that the muscle group for
each channel is noted
Data-logging is required
Use EMG Channels, Trigger Output and Stimulation Current Record
to log activity as required using as oscilloscope or chart recorder

Neurosign 100
Operating Manual
© The Magstim Company Limited 13 NOP01-EN-02
SECTION 5MAINTENANCE
5.1 User Maintenance and Calibration
There are no user maintenance or calibration requirements for the Neurosign 100 and accessories.
However, it is important that they are checked for any sign of damage at the start of each session. If
any cracks are visible in the plastics, or if there is damage to any of the cables, the items must not be
used and should be returned to The Magstim Company Limited for servicing and repair (see Section
7.2 for contact details).
5.2 Voltage Selection and Fuse Rating
The Neurosign 100 is preset at the factory to operate at 100V, 120V, 220V or 240V A.C., as
appropriate. The following are the fuse ratings:
Supply Voltage Qty Fuse Size Rating
100-120V~ 2 20 x 5mm T630mA 250V
220-240V~ 2 20 x 5mm T315mA 250V
The fuses may be changed by opening the fuse cover, pulling out the fuse trays (marked with an
arrow), replacing the blown fuse with one of the correct rating, and by locating and pushing home the
trays. Replace the fuse cover.
The voltage may be selected by removing the fuse cover, rotating the selector and replacing the
cover. A legend will indicate the voltage selected when the fuse cover is replaced.
Only replace fuses with those of the correct rating, and only operate the instrument with the
voltage selector set at the appropriate voltage. Failure to do so may cause serious damage to
the device(s).
5.3 Cleaning and Disinfecting
The Neurosign 100 and accessories cannot be sterilised; therefore, do not allow them to become
contaminated with bodily fluids. They may be cleaned using an isopropyl-moistened cloth but ensure
that they have dried thoroughly before use.
5.4 Servicing
Servicing of Neurosign 100 and accessories must only be carried out by The Magstim Company
Limited or one of its authorised service centres. To arrange a return or for further information, contact
the service department at The Magstim Company Limited (see Section 7.2 for contact details).
5.5 Device Lifetime
The lifetime of the Neurosign 100, Stimulator Probe Pod and Pre Amplifier Pod are defined as being
five years from the date of shipment. The Magstim Company Limited will support the products for the
duration of their lifetime.
5.6 Disposal
When a Neurosign 100 or accessory reaches the end of its serviceable life, The Magstim Company
Limited should be contacted (see Section 7.2 for contact details) to arrange for its disposal in
compliance with the appropriate environmental regulations.
Single use disposable accessories should be disposed of immediately following use using a “sharps”
disposal box.

Neurosign 100
Operating Manual
© The Magstim Company Limited 14 NOP01-EN-02
SECTION 6SPECIFICATIONS
6.1 Safety Specifications
During use, the type of protection against electric shock provided by the Neurosign 100 System is
classified as Class 1.
The degree of protection against electric shock for applied part accessories is classified as Type BF
Applied Parts. This means that the Stimulating Probes, Stimulator Pod circuits, Electrodes and Pre-
amplifier Pod circuits are electrically isolated from the other parts of the equipment and meet Type BF
leakage current limits as required by IEC 60601-1.
The Neurosign 100 and accessories comply with the requirements of Safety Standard IEC 60601-1
and with EMC Standard EN 60601-1-2.
To avoid problems with EMC interference, the Neurosign 100 and accessories should not be used in
the vicinity of any equipment that does not comply with EMC Safety Standard EN 60601-1-2,
including mobile phones. Any interface cable which connects the Neurosign 100 unit to an external
piece of equipment must be no more than 1.5m in length.
Only equipment that meets the relevant IEC standards and is configured in compliance with IEC
60601-1-1, should be connected to the Neurosign 100.
The Neurosign 100 and accessories are classified as IPX0 (Not Protected), as there is no specialised
protection provided against the ingress of liquids.
The Neurosign 100 and accessories are not protected against flammable anaesthetic mixtures. They
are not suitable for use in the presence of a flammable anaesthetic mixture with air, oxygen or nitrous
oxide.
The mode of operation of the Neurosign 100 and accessories is classified as continuous.

Neurosign 100
Operating Manual
© The Magstim Company Limited 15 NOP01-EN-02
6.2 Technical Specifications
Stimulator Output
Current Output: Constant,
50µA –5mA ±20% or ±25µA, whichever is greater
Stimulator Frequency: 30Hz or 3Hz
Waveform: 200µs pulse width, negative going pulse
Isolation: 1.5kV r.m.s. to ground
Amplifier
Channels: 2, Input noise of 8µV peak to peak,
CMRR greater than 95dB
Frequency range: 10Hz to 10kHz ± 3dB
Isolation: 1.5kV r.m.s. to ground
Audio compression ratio: 10:1
Channel ident: Tuned filter system
Speaker: 165mm
Amplifier output: 14W
Display
Bargraph: Twin 20 segment logarithmic, trailing segment
Range: 30µV to 20mV ±20% peak to peak
Indicators: Channel 1 and 2 on-off indicators
Channel 1 and 2 artefact warning indicators
Stimulation Current flow current confirm indicator
Output Ports
Dual EMG output: Gain of 500
Headphone socket: >16 ohm impedance, mono
Stimulation Current Record: 1V/mA for data logging purposes
Trigger output: TTL level
Power Supply
Power: 220-240/100-120V~ 50-60Hz; 50VA
Fuse(s): 2 x T630mA 20x5mm for 100-120V~
2 x T315mA 20x5mm for 220-240V~
General Specifications
Dimensions: 290mm x 260mm x 100mm
Weight: 6kg
Software: Neurosign 100 Bar graph - Revision 00
(Software ref: 1682)
Please note: All specifications are subject to alteration.

Neurosign 100
Operating Manual
© The Magstim Company Limited 16 NOP01-EN-02
6.3 Environmental Conditions
Transport and storage temperature: -19°C to 60°C
Transport and storage relative humidity: 10% to 80% (non-condensing)
Operating temperature: 5°C to 30°C
Operating relative humidity: 30% to 70% (non-condensing)
Atmospheric pressure: 50kPa to 106kPa
6.4 Packing Instructions
If, for any reason, it is necessary to return your Neurosign 100 unit care should be taken to ensure
that the equipment is adequately packed to prevent transit damage. Ideally the equipment should be
returned in its original packing. If this or an adequate replacement is not available, replacement
shipping cartons can be obtained from the Magstim Company Limited.
The Neurosign 100 System must be completely disconnected before shipping. Failure to do so is
likely to result in transit damage to the casing.

Neurosign 100
Operating Manual
© The Magstim Company Limited 17 NOP01-EN-02
SECTION 7CONTACT DETAILS
7.1 Product Enquiries
The Magstim Company Limited
Spring Gardens, Whitland, Carmarthenshire, SA34 0HR
Telephone: +44 (0)1994 240798
Fax: +44 (0)1994 240061
E-mail: [email protected]
Website: www.neurosign.com
7.2 Servicing Enquiries
Telephone: +44 (0)1994 242900
Fax: +44 (0)1994 240061
E-mail: service@neurosign.com
7.3 Sales Enquiries
Telephone: +44 (0)1994 241111
Fax: +44 (0)1994 242917
E-mail: [email protected]
Neurosign® is a registered trademark of The Magstim Company Limited in the UK and USA
Table of contents