mammotome REVOLVE Operation and maintenance manual
USER INSTRUCTIONS AND
OPERATIONS GUIDE
www.mammotome.com/ifu eIFU number: 001413

Table of Contents
CHAPTER 1INTRODUCTION...................................................................................................................................1-1
Indications...........................................................................................................................................................1-1
Contraindications................................................................................................................................................1-1
Warnings .............................................................................................................................................................1-1
General............................................................................................................................................................1-2
System.............................................................................................................................................................1-2
Control Module...............................................................................................................................................1-3
Holster.............................................................................................................................................................1-3
Probe...............................................................................................................................................................1-3
Training ...............................................................................................................................................................1-4
System Components ...........................................................................................................................................1-4
CHAPTER 2SYSTEM DESCRIPTION......................................................................................................................2-1
Overview—System Description ..........................................................................................................................2-1
Control Module...............................................................................................................................................2-3
Cart ................................................................................................................................................................2-3
EX Holders (Cart Detachable Component)......................................................................................................2-4
ST Probe and Vacuum Tube Set......................................................................................................................2-4
U/S Probe and Vacuum Tube Set....................................................................................................................2-7
EX Probe, Vacuum Tube Set, and Sleeve ........................................................................................................2-9
Input Devices ................................................................................................................................................2-11
Detachable Components ..............................................................................................................................2-19
System Software ...........................................................................................................................................2-20
CHAPTER 3INITIAL INSTALLATION AND GETTING STARTED................................................................................3-1
Preventative Maintenance..................................................................................................................................3-1
Unpacking/Assembling the Mammotome revolve Control Module...................................................................3-1
Unpacking the Cart..............................................................................................................................................3-2
Unpacking the EX Holder (Cart Detachable Component)...................................................................................3-2
Placing the Control Module on the Cart .............................................................................................................3-2
Unpacking the Holster.........................................................................................................................................3-3
Unpacking the Remote Footswitch.....................................................................................................................3-3
Unpacking the Remote Keypad...........................................................................................................................3-3
Setup ...................................................................................................................................................................3-3

1. Powering on the Control Module...........................................................................................................3-3
2. Connecting the Holster to the Control Module......................................................................................3-4
3. (OPTIONAL) Attaching the EX Holder (Cart Detachable Component) to the Cart..................................3-6
4. (OPTIONAL) Connecting the Remote Footswitch to the Control Module..............................................3-6
5. (OPTIONAL) Connecting the Remote Keypad to the Control Module....................................................3-6
6. Connecting the Vacuum Canister to the Control Module......................................................................3-6
7. (OPTIONAL –EX ONLY) Connecting the Sleeve to the Mammotome revolve EX Holster......................3-7
8. Loading the Mammotome revolve Probe onto the Mammotome revolve Holster...............................3-8
9. Connecting the Probe Vacuum Tube Set to the Mammotome revolve Dual Vacuum-Assisted Biopsy
System ..................................................................................................................................................3-13
10. Holster/Probe Assembly Initialization..................................................................................................3-15
11. Select Side of Holster and Ready for Procedure (ST Only) ...................................................................3-16
Chapter 4 STEREOTACTIC (ST) INSTRUCTIONS FOR USE .................................................................................4-1
Overview .............................................................................................................................................................4-1
Mammotome revolve ST Probe and ST Holster..................................................................................................4-1
Preparation for Use.............................................................................................................................................4-3
Instructions for Use.............................................................................................................................................4-3
Steps for Tissue Sampling ...............................................................................................................................4-3
Other Instructions...........................................................................................................................................4-7
Disassembling the Mammotome revolve Dual Vacuum-Assisted Biopsy System..........................................4-9
Shutting down the Mammotome revolve Dual Vacuum-Assisted Biopsy System .......................................4-11
Chapter 5 ULTRASOUND (U/S) INSTRUCTIONS FOR USE...................................................................................5-1
Overview .............................................................................................................................................................5-1
Mammotome revolve U/S Probe and U/S Holster .............................................................................................5-1
Preparation for Use.............................................................................................................................................5-2
Instructions for Use.............................................................................................................................................5-3
Steps for Tissue Sampling ...............................................................................................................................5-3
Other Instructions...........................................................................................................................................5-5
Disassembling the Mammotome revolve Biopsy System...............................................................................5-7
Disconnecting Input Devices...........................................................................................................................5-8
Shutting Down the Mammotome revolve Dual Vacuum-Assisted Biopsy System.........................................5-8
Chapter 6 EX INSTRUCTIONS FOR USE ..............................................................................................................6-1
Overview .............................................................................................................................................................6-1
Mammotome revolve EX Probe and EX Holster .................................................................................................6-1
Preparation for Use.............................................................................................................................................6-2

Instructions for Use.............................................................................................................................................6-2
Steps for Tissue Sampling ...............................................................................................................................6-2
Other Instructions...........................................................................................................................................6-5
Disassembling the Mammotome revolve Biopsy System...............................................................................6-7
Shutting Down the Mammotome revolve Dual Vacuum-Assisted Biopsy System.......................................6-10
CHAPTER 7CLEANING AND DISINFECTION..........................................................................................................7-1
Introduction ........................................................................................................................................................7-1
Cleaning Instructions for the Control Module, Cart, and Remote Footswitch...................................................7-1
Cleaning and Disinfection Instructions for the Holsters and Remote Keypad....................................................7-2
Cleaning the ST or U/S Holster: Option 1 .......................................................................................................7-2
Cleaning the ST or U/S Holster: Option 2 .......................................................................................................7-2
Disinfecting the ST or U/S Holster: Option 1 ..................................................................................................7-3
Disinfecting the ST or U/S Holster: Option 2 ..................................................................................................7-3
Cleaning the EX Holster...................................................................................................................................7-4
Disinfecting the EX Holster .............................................................................................................................7-4
Cleaning the Holster Holder............................................................................................................................7-4
Cleaning the Remote Keypad: Option 1..........................................................................................................7-5
Cleaning the Remote Keypad: Option 2..........................................................................................................7-5
Disinfecting the Remote Keypad: Option 1 ....................................................................................................7-5
Disinfecting the Remote Keypad: Option 2 ....................................................................................................7-6
Chapter 8 SOFTWARE DESCRIPTION...................................................................................................................8-1
Touchscreens and Screen Button Functions.......................................................................................................8-1
Device Confirmation and Initialization Screens..............................................................................................8-1
ST and U/S Procedure Screens........................................................................................................................8-3
EX Procedure Screens .....................................................................................................................................8-9
Standby Screens............................................................................................................................................8-13
Utilities Screens ............................................................................................................................................8-14
CHAPTER 9SERVICE AND TROUBLESHOOTING...................................................................................................9-1
Service.................................................................................................................................................................9-1
Contact Information............................................................................................................................................9-8
Chapter 10 SYSTEM SPECIFICATIONS ..............................................................................................................10-1
Classification .....................................................................................................................................................10-1
Storage/Operating Conditions..........................................................................................................................10-2
Electrical Specifications.....................................................................................................................................10-2
System Specifications........................................................................................................................................10-5

RFID Frequency Information.............................................................................................................................10-6
WEEE (The Waste Electrical and Electronic Equipment Directive ....................................................................10-7
All Other Symbol and Labeling Information......................................................................................................10-7
How Supplied ....................................................................................................................................................10-7
Responsibility of the Manufacturer..................................................................................................................10-7
Calling for Service..............................................................................................................................................10-8
Requesting a Paper Copy of the Information for Use (IFU) ..............................................................................10-8
Additional Product Information........................................................................................................................10-8
Chapter 11 WARRANTY......................................................................................................................................11-1
Chapter 12 STEREOTACTIC (ST) HOLSTER STATES.........................................................................................12-1

1-1
Chapter 1 INTRODUCTION
Please read all information carefully.
WARNING: FAILURE TO FOLLOW THE INSTRUCTIONS MAY LEAD TO SERIOUS SURGICAL
CONSEQUENCES.
All information in this guide applies to Stereotactic Guided Procedures (ST) and Ultrasound Guided
Procedures (U/S, EX) unless otherwise stated.
IMPORTANT: This document is designed to provide instructions for use of the Mammotome revolve Dual
Vacuum-Assisted Biopsy System. It is not a reference to surgical technique. Access to the
internet and a .pdf viewer is required to view the full electronic Instructions for Use.
NOTE:The information in this guide is subject to change without notice.
Indications
The Mammotome revolve Dual Vacuum Assisted Biopsy (VAB) System is indicated to provide tissue samples for
diagnostic sampling of breast abnormalities.
•The Mammotome revolve Dual Vacuum Assisted Biopsy (VAB) System is intended to provide breast tissue
for histologic examination with partial or complete removal of the imaged abnormality.
•The Mammotome revolve Dual Vacuum Assisted Biopsy (VAB) System is intended to provide breast tissue
for histologic examination with partial removal of a palpable abnormality.
The extent of a histologic abnormality cannot always be readily determined from palpation or imaged appearance.
Therefore, the extent of removal of the palpated or imaged evidence of an abnormality does not predict the extent
of removal of a histologic abnormality, e.g., malignancy. When the sampled abnormality is not histologically
benign, it is essential that the tissue margins be examined for completeness of removal using standard surgical
procedures.
In instances when a patient presents with a palpable abnormality that has been classified as benign through
clinical and/or radiological criteria (e.g., fibroadenoma, fibrocystic lesion), the Mammotome revolve Dual Vacuum
Assisted Biopsy (VAB) System may also be used to partially remove such palpable lesions. Whenever breast
tissue is removed, histological evaluation of the tissue is the standard of care. When the sampled abnormality is
not histologically benign, it is essential that the tissue margins be examined for completeness of removal using
standard surgical procedures.
Contraindications
•This device is not intended for use except as indicated.
•The instrument is contraindicated for those patients where increased risk or complications may be
associated with the percutaneous removal of tissue samples based upon the physician’s judgment.
Patients receiving anticoagulant therapy or who may have bleeding disorders may be at increased risk.
Warnings
Please read all the contents of the User Instructions and Operations Guide for your Mammotome revolve Dual
Vacuum-Assisted Biopsy System prior to installation and operation. Follow all warnings and instructions as stated
in this guide and retain this guide for future reference.

1-2
General
othe Mammotome revolve Dual Vacuum-Assisted Biopsy System is used in the presence of
flammable anesthetics. Avoid at all costs.
oAs with any medical procedure, there is potential risk for infection.
oMinimally invasive instruments may vary in dimensions (e.g., diameter) from manufacturer to
manufacturer. When minimally invasive instruments and accessories from different manufacturers
are employed together in a procedure, verify compatibility prior to initiation of the procedure.
oMinimally invasive procedures should be performed only by persons having adequate training
and familiarity with minimally invasive techniques. Consult medical literature relative to
techniques, complications, and hazards prior to performance of any minimally invasive procedure.
System
oTo avoid the risk of electrical shock, this equipment must only be connected to a supply mains
with protective earth.
oChanges or modifications not expressly approved by the party responsible for compliance could
void the user’s authority to operate the equipment.
oThis medical device emits electromagnetic energy that may interfere with other nearby medical
devices, which may cause those devices to malfunction or seriously harm the patient.
oDo not use the Mammotome revolve Dual Vacuum-Assisted Biopsy System in an oxygen-rich
environment due to a small risk of fire. Avoid at all costs.
oDo not use this instrument in conjunction with Magnetic Resonance Imaging (MRI).
oThis instrument should be used only by physicians trained in percutaneous needle techniques for
tissue collection.
oDo not attempt to sterilize the holsters, control module, cart, remote keypad, or remote footswitch
through autoclave, ethylene oxide, radiation, or plasma sterilization procedures. Do not spray with
fluids or submerge in fluids; this may damage the instrument. Improper cleaning may void the
warranties.
oDo not process the device through an automated washer-disinfect or ultrasound bath.
oMinimally invasive procedures should be performed only by persons having adequate training
and familiarity with minimally invasive techniques. Consult medical literature relative to
techniques, complications, and hazards prior to performance of any minimally invasive procedure.
oDo not immerse electrosurgical instruments in liquid unless the instruments are designed and
labeled to be immersed.
oThe Mammotome revolve Dual Vacuum Assisted Biopsy System is not suit able for use in
environments that contain a mixture of flammable anesthetic and air or oxygen and nitrous oxide.
oDo not remove the vacuum tubing from the vacuum canister when the vacuum is being used by
the system.
oProducts manufactured or distributed by companies not authorized by Devicor Medical Products,
Inc. may not be compatible with the Mammotome revolve Dual Vacuum-Assisted Biopsy System.
Use of such products may lead to unanticipated results and possible injury to the user or patient.

1-3
oAttempts to disassemble or service any Mammotome product other than in the manner described
in the product’s accompanying labeling, or by anyone other than a qualified technician, may
cause injury because of electrical shock.
oAll appropriate surgical rom safety precautions should be observed and implemented before and
during the tissue sampling procedure.
oInspect the power cords on the holster, remote footswitch, and remote keypad for fraying or
damage prior to each use. Do not use the component if it shows signs of damage or wear.
oDo not touch exposed parts of connectors on the control module, holster, or touchscreen while
simultaneously contacting the patient, as safety hazards may exist.
oEnsure that all cords are clear of the cart wheels prior to transporting the system.
oDo not attempt to open or access internal components of the Mammotome revolve Dual Vacuum-
Assisted Biopsy System, as safety hazards may exist.
oNo modification of this equipment is allowed.
oDo not modify this equipment without the manufacturer’s authorization. If this equipment is
modified, appropriate inspection and testing must be conducted to ensure the continued safe use
of equipment.
oThe pressure differential created in the canister during the procedure can cause blood discharge.
oThis medical device emits electromagnetic energy that may interfere with other nearby medical
devices, which may cause those devices to malfunction or serious injury to patient.
Control Module
oTo ensure adequate ventilation, position the control module at least 6" (15.24 cm) from
all walls. Do not block the exhaust fan at the rear of the control module. Do not position
the device where it is difficult to operate the mains disconnect device. The power switch
is used for mains disconnect.
oDo not use if the Control Module is damaged. Electric shock is possible.
oTo avoid the risk of electrical shock, this equipment must only be connected to a supply mains
with protective earth."
Holster
oWhen system power is on, keep the holster cavity clear of all foreign objects.
oDuring use, ensure the electric cable is not pinched or bound. The cable should be free of kinks
or stress for proper operation.
Probe
oDo not reuse, reprocess, or resterilize the probe. The device is packaged and sterilized for single
use only. Reuse, reprocessing, or resterilization may compromise the structural integrity of the
device and/or lead to device failure, which may result in patient injury, illness, or death. In
addition, reprocessing or resterilization of single use devices may create a risk of contamination
and/or cause patient infection or cross-infection, including, but not limited to, the transmission of
infectious disease(s) from one patient to another. Contamination of the device may lead to injury,
illness, or death of the patient.
oEnsure that the connection tubes are not placed over the docked handpiece.
1-4
oAvoid scraping of the needle tip against the protective sleeve should remain on the probe needle
until you are ready to perform the tissue sampling procedure.
oand injury while removing the protective sleeve.
oDisconnect the saline spike from the saline bag. Discard the saline bag using standard medical
techniques after each patient use.
oKeep hands clear of the sample aperture and probe needle tip at all times.
oDo not probe tubing pathways should be free from debris during use.
oTake special care to avoid bending or twisting the probe.
oIf the probe is bent, do not use. Dispose of the probe in the appropriate container.
oInstruments or devices that come into contact with bodily fluids may require special disposal
handling to prevent biological contamination.
oWhen placing or removing the holster/probe assembly to or from the docking station on the cart,
ensure no contact is made between sterile and non-sterile surfaces (e.g., needle tip to cart
surfaces or control module).
Training
New Installations
In-service training is provided at the customer's site.
Repeat Training and Clinical Application Consultation
Repeat of in-service training and clinical application consultation is available at the customer’s request. (See
Chapter 9, Service and Troubleshooting, Contact Information.)
System Components
Only the devices identified below are designed for compatible functionality with the system. The Mammotome
revolve Biopsy System comprises the following components:
Mammotome revolve EX System (for Ultrasound Guided Procedures)
•Mammotome revolve Control Module
•Mammotome revolve Cart
•Mammotome revolve EX Holster
•Mammotome revolve EX Probe (provided with or without Holster sleeve)
•Mammotome revolve Remote Footswitch
•Mammotome revolve Remote Keypad
•Mammotome revolve System Software
oThe Mammotome revolve EX Holster and EX Probes are only compatible with Mammotome revolve
System Software.
•Bemis800cc Vacuum Canister (Manufactured for use with the Mammotome revolve dual Vacuum-
Assisted Biopsy System)
•Mammotome revolve EX Holders (Cart Detachable Component)
Mammotome revolve ST System (for Stereotactic Guided Procedures)
•Mammotome revolve Control Module

1-5
•Mammotome revolve Cart
•Mammotome revolve ST Holster
•Mammotome revolve ST Probe (with attached Specimen Management System and Vacuum Tube Set)
•Mammotome revolve Remote Footswitch
•Mammotome revolve Remote Keypad
•Mammotome revolve System Software
oThe Mammotome revolve ST Holster and ST Probes are only compatible with Mammotome revolve
System Software.
•Bemis800cc Vacuum Canister (Manufactured for use with the Mammotome revolve dual Vacuum-
Assisted Biopsy System)
•Mammotome revolve Specimen Management System(s)
•Mammotome revolve Probe Guide(s)
Mammotome revolve U/S System (for Ultrasound Guided Procedures)
•Mammotome revolve Control Module
•Mammotome revolve Cart
•Mammotome revolve U/S Holster
•Mammotome revolve U/S Probe (with attached Specimen Management System and Vacuum Tube Set)
•Mammotome revolve Remote Footswitch
•Mammotome revolve Remote Keypad
•Mammotome revolve System Software
oThe Mammotome revolve U/S Holster and U/S Probes are only compatible with Mammotome revolve
System Software.
•Bemis800cc Vacuum Canister (Manufactured for use with the Mammotome revolve Biopsy System)
•Mammotome revolve Specimen Management System(s)

2-1
Chapter 2 SYSTEM DESCRIPTION
All information in this guide applies to ST, U/S, and EX modalities, unless otherwise stated.
Overview—System Description
The Mammotome revolve Dual Vacuum-Assisted Biopsy System allows the user to sample breast tissue that has
been identified as suspicious under imaging guidance. Multiple tissue samples can be taken without removing the
needle. The user may identify the tissue as it is collected to correspond with the imaging display.
The Mammotome revolve Dual Vacuum-Assisted Biopsy System comprises three primary subsystems:
1. Control Module: a reusable control module, containing the vacuum pump, power supply, valve
actuators, user touchscreen interface, and control electronics.
2. Holster: a reusable holster, containing the drive motors, gear trains, and user activation buttons.
3. Probe: a disposable single-patient use probe, containing the biopsy needle, specimen management
system, and vacuum tube set.
In addition, several optional reusable detachable components are available including a transport cart, remote
footswitch, and remote keypad controls. Disposable detachable components required include a vacuum canister
(ST, U/S, and EX), probe guides (ST only) and a probe and holster holder (EX only). A disposable Holster
Sleeve (EX only) for single-patient use, provided with a packaged EX Probe, is intended to provide a barrier
between the user’s gloved hand and reusable Holster.
NOTE: The usable volume of the canister is 800cc.
Fluids can be delivered through the Mammotome revolve probe for managing selected patient/procedure
requirements.
Biopsy site identifiers may be used in conjunction with the ST and U/S probes to radiographically mark the
location of the biopsy procedure. In the event of removal of evidence of the abnormality, it is strongly
recommended that a biopsy site identifier or other marking device be placed to mark the biopsy site (in case
follow-up is required).
NOTE: Biopsy site identifiers are not intended to be placed through the EX probe. Alternative methods should be
used if placement of a biopsy site identifier is required.
NOTE: Refer to the Biopsy Site Identifier Instructions for Use for compatibility, confirmed fit, function, and length
with the Mammotome revolve Dual Vacuum-Assisted Biopsy System.

2-2
The Mammotome revolve Dual Vacuum-Assisted Biopsy System comprises the following components and input
devices:
Figure 1. Mammotome revolve Dual Vacuum-Assisted Biopsy System Components

2-3
Control Module
The Control Module is an electronic device that runs the software necessary to operate the Mammotome
revolve Dual Vacuum-Assisted Biopsy System. The device also houses the vacuum pump, power supply,
valve actuators, user touchscreen interface, and control electronics.
Figure 2. Control Module Illustration and Nomenclature
Cart
The cart is designed exclusively for use with Mammotome revolve Dual Vacuum-Assisted Biopsy System and
is used for moving the control module in the clinical environment.
NOTE: The working load of the Mammotome revolve cart is equivalent to the mass of the Mammotome
revolve components (control module, footswitch, remote control, ST holster and holder, EX holster
and holder), which is 34 lbs. The total mass of the Mammotome revolve cart loaded with the
Mammotome revolve system is 75 lbs.

2-4
Figure 3. Cart Illustration and Nomenclature
EX Holders (Cart Detachable Component)
The EX holders are designed exclusively for use with the Mammotome revolve Cart to contain the
Mammotome revolve EX Holster and/or EX Probe in the clinical environment.
Figure 4.: EX Holders Illustration
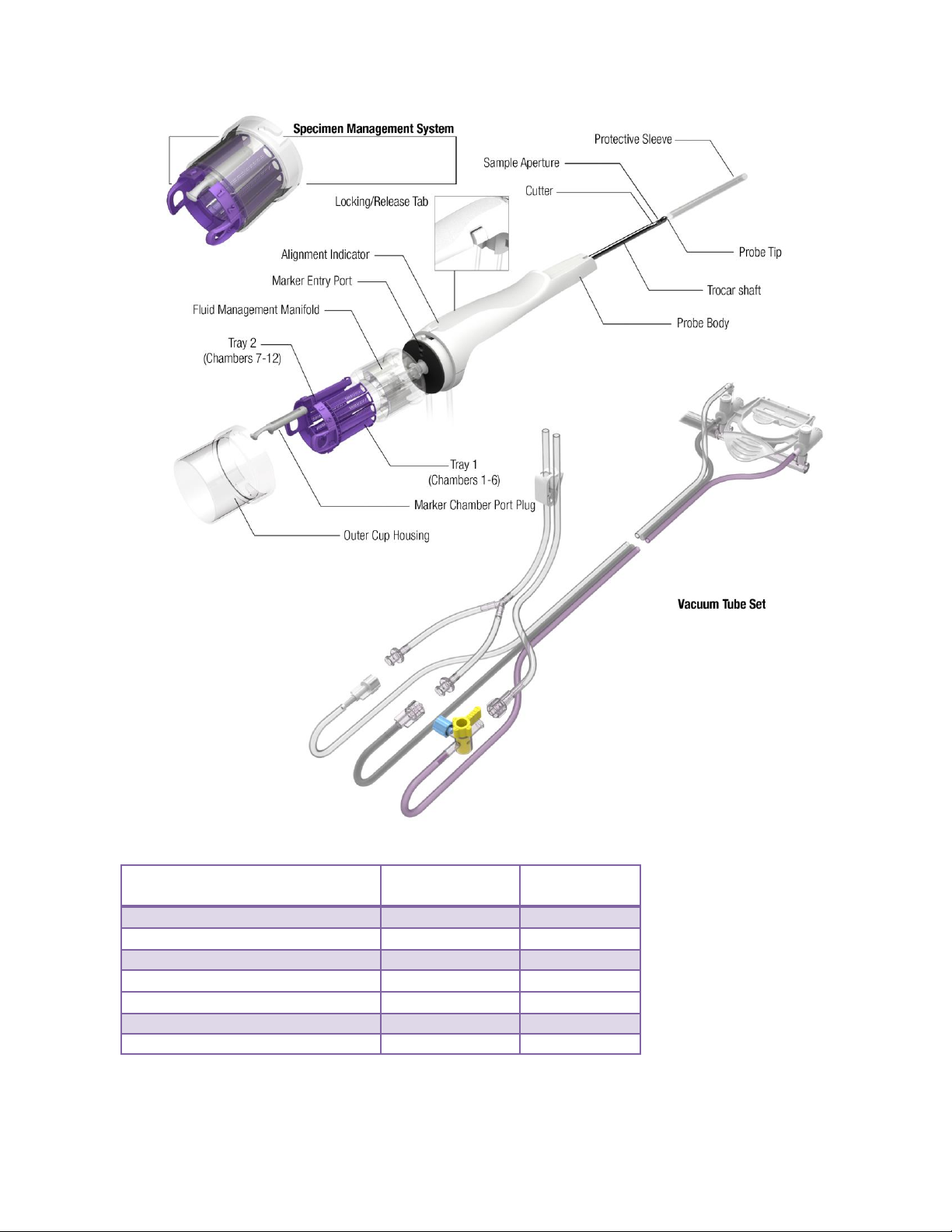
ST Probe and Vacuum Tube Set
The ST Probe (along with its integrated components: Specimen Management System and Vacuum
Tube Set) is a sterile, single-patient use device that may be used with imaging guidance to excise a
tissue sample for diagnosis. The probe is designed to be loaded onto the ST holster. The body of the
probe contains locking tabs to secure the probe to the ST holster. The probe consists of an outer
trocar shaft and an inner cutter in a distal sample aperture. The sample aperture can be rotated to the
desired orientation using either the probe’s aperture rotation thumbwheel or the ST Holster aperture
rotation knobs.
The probe comes packaged with an attached vacuum tube set. The vacuum tube set connects the
probe to a vacuum source through the surgical side of a vacuum canister and allows fluid collected
from the probe to be drained into the canister. The tube set has three points of attachment: 1) to the
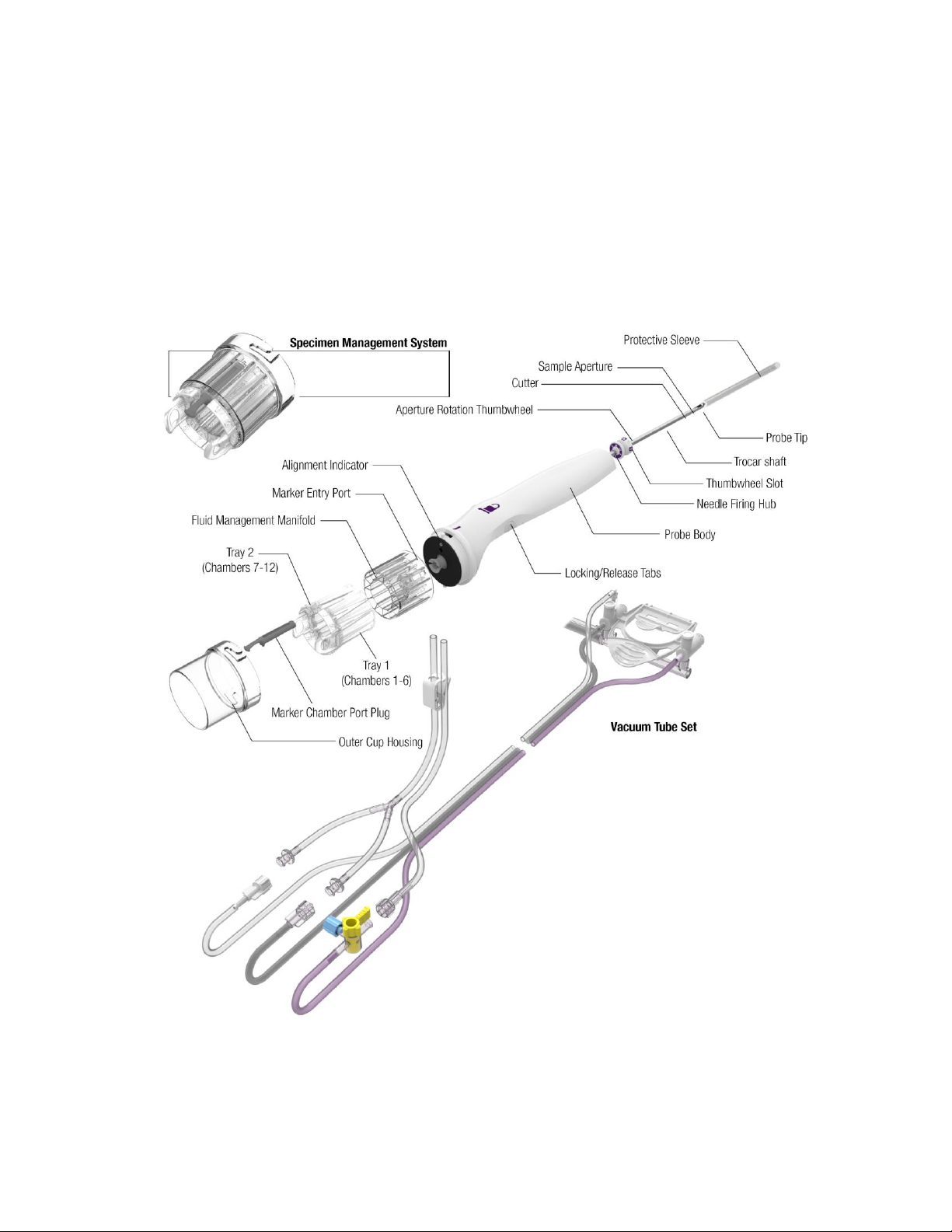
2-5
control module vacuum connection slot, 2) to the vacuum canister port, and 3) to a saline bag.
For further convenience, the probe includes a Specimen Management System consisting of 12
proximal specimen collection chambers. Each collection chamber is designed to capture and hold
excised tissue. The specimen collection chambers are designed to be removed from the fluid
management manifold in two separate trays (of six chambers each). If desired, the tissue can remain
in the chambers to be directly imaged in the post-biopsy specimen radiograph.
A biopsy site identifier may be used in conjunction with the Mammotome revolve probes to
radiographically mark the location of the biopsy procedure. In the event of removal of evidence of
abnormality, it is strongly recommended that a marking device be placed to mark the biopsy site in
case follow-up is required.
Figure 5. ST Probe and Vacuum Tube Set Illustration and Nomenclature
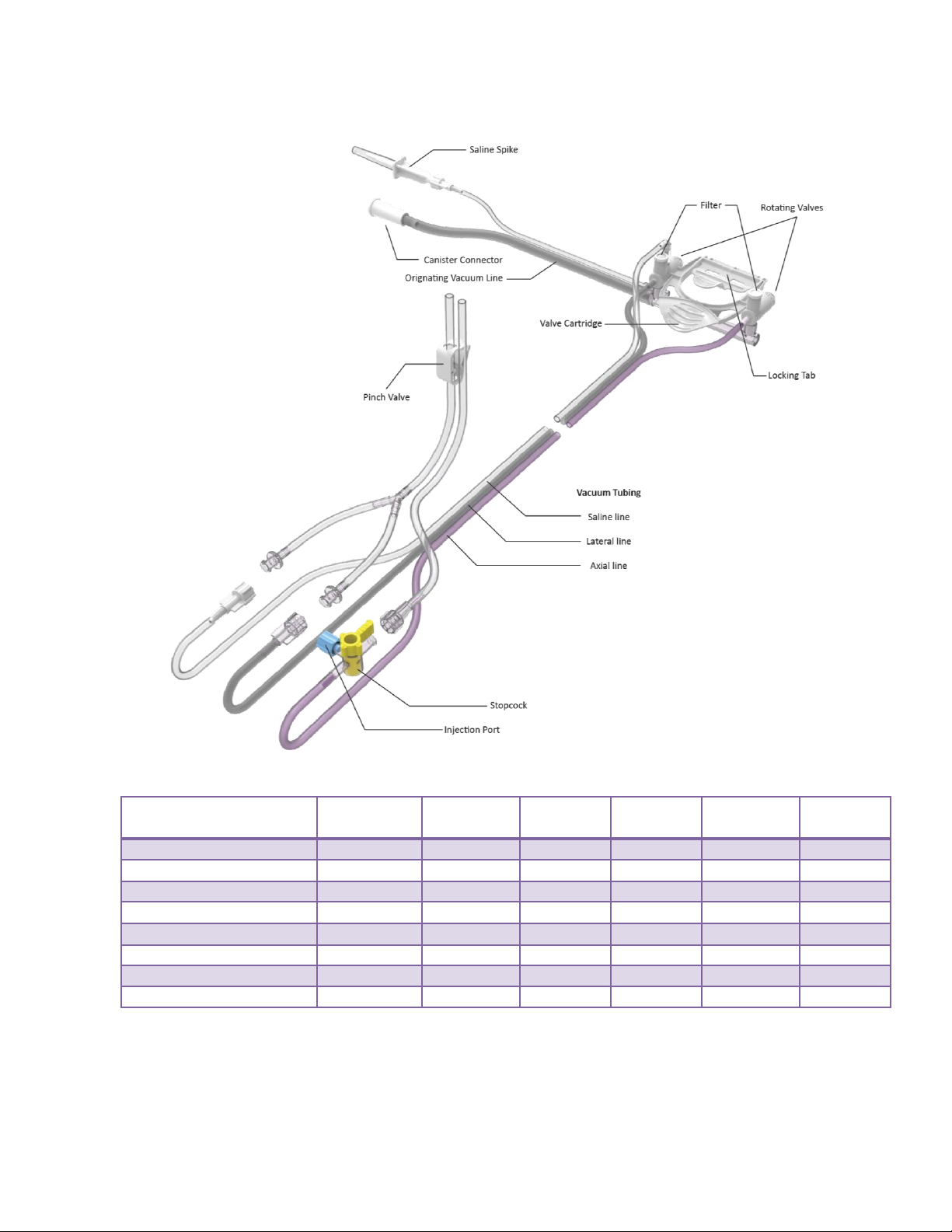
2-6
Figure 6. Vacuum Tube Set Illustration and Nomenclature
Table 1. Mammotome revolve ST Probe Specifications
Dimension (mm)
8G 9cm
MST0809
8G 12cm
MST0812
8G 15cm
MST0815
10G 9cm
MST1009
10G 12cm
MST1012
10G 15cm
MST1015
A: Needle Height
5.6
5.6
5.6
4.9
4.9
4.9
B: Needle Width
4.4
4.4
4.4
3.45
3.45
3.45
C: Tip to Center
21.0
21.0
21.0
17.5
17.5
17.5
D: Tip to Thumbwheel
91.5
121.5
151.5
90.0
120.0
150.0
E: Dead Space
9.5
9.5
9.5
8.0
8.0
8.0
F: Aperture Length
23.0
23.0
23.0
19.05
19.05
19.05
Fill Volume (cc)
9
9
10
8
8
8
Firing Distance
19.3
19.3
19.3
19.3
19.3
19.3
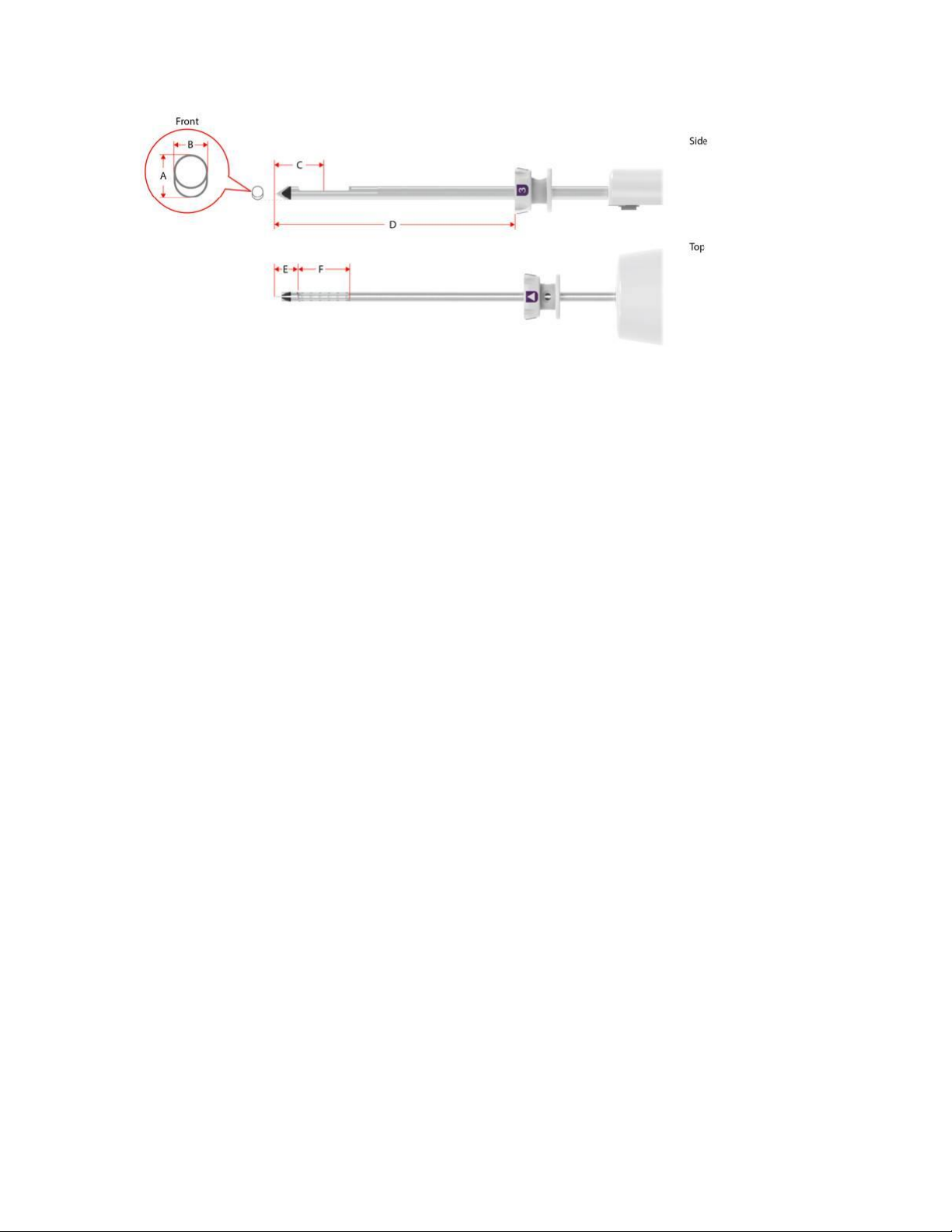
2-7
Figure 7. Mammotome revolve ST Probe Illustrations
U/S Probe and Vacuum Tube Set
The U/S Probe (along with its integrated components: Specimen Management System and Vacuum
Tube Set) is a sterile, single-patient use device that may be used with imaging guidance to excise a
tissue sample for diagnosis. The probe is designed to be loaded onto the U/S holster. The body of the
probe contains a locking tab to secure the probe to the U/S holster. The probe consists of an outer
trocar shaft and an inner cutter in a distal sample aperture. The sample aperture can be manually
rotated by using the wrist to rotate the probe/holster assembly to the desired orientation.
The probe comes packaged with an attached vacuum tube set. The vacuum tube set connects the
probe to a vacuum source through the surgical side of a vacuum canister. This allows fluid collected
from the probe to be drained into the canister. The tube set has three points of attachment: 1) to the
control module vacuum connection slot, 2) to the vacuum canister port, and 3) to a saline bag.
For further convenience, the probe includes a Specimen Management System consisting of 12
proximal specimen collection chambers. Each collection chamber is designed to capture and hold
excised tissue. The specimen collection chambers are designed to be removed from the fluid
management manifold in two separate trays (of six chambers each). If desired, the tissue can remain
in the chambers to be directly imaged in the post-biopsy specimen radiograph.
A biopsy site identifier may be used in conjunction with the Mammotome revolve U/S probes to mark
the location of the biopsy procedure. In the event of removal of evidence of abnormality, it is strongly
recommended that a marking device be placed to mark the biopsy site in case follow-up is required.
2-8
Figure 8. U/S Probe and Vacuum Tube Set Illustration and Nomenclature
Table 2. Mammotome revolve U/S Probe Specifications
Dimension (mm)
8G
MHUS08
10G
MHUS10
A: Needle Height
5.6
4.9
B: Needle Width
4.4
3.45
C: Tip to Center
21.0
17.5
D: Needle Length
123.55
124.04
E: Dead Space
9.5
8.0
F: Aperture Length
23.0
19.05
Fill Volume (cc)
9
8
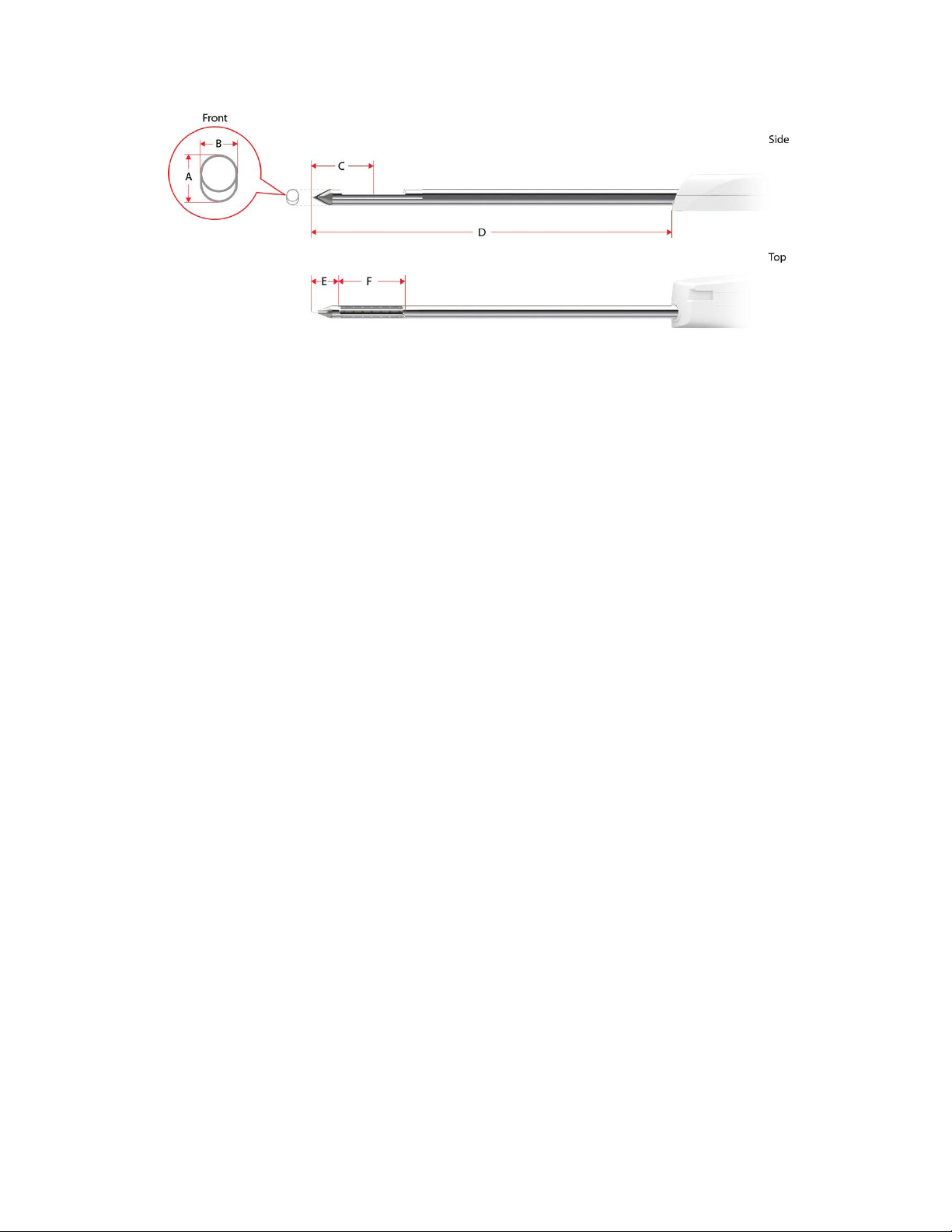
2-9
Figure 9. Mammotome revolve U/S Probe Illustrations
EX Probe, Vacuum Tube Set, and Sleeve
The EX Probe (along with its integrated components: Specimen Management System, Vacuum Tube
Set, and Optional Sleeve) is a sterile, single-patient use device that may be used with imaging
guidance to excise a tissue sample for diagnosis. The probe is designed to be loaded onto the EX
holster. The body of the probe contains a locking tab to secure the probe to the EX holster. The probe
consists of an outer trocar shaft and an inner cutter in a distal sample aperture. The sample aperture
can be manually rotated by using the wrist to rotate the probe/holster assembly to the desired
orientation.
The probe comes packaged with an attached vacuum tube set. The vacuum tube set connects the
probe to a vacuum source through the surgical side of a vacuum canister. This allows fluid collected
from the probe to be drained into the canister. The tube set has three points of attachment: 1) to the
control module vacuum connection slot, 2) to the vacuum canister port, and 3) to a saline bag.
For further convenience, the probe includes a Specimen Management System consisting of 4 proximal
specimen collection chambers. Each collection chamber is designed to capture and hold excised
tissue. The specimen collection chambers are designed to be removed from the fluid management
manifold in four separate trays, three large and one single tray.
The MHEX08S EX probe comes packaged with a Holster sleeve. The sleeve connects to the holster
and covers the reusable EX Holster and a portion of the Holster cable.
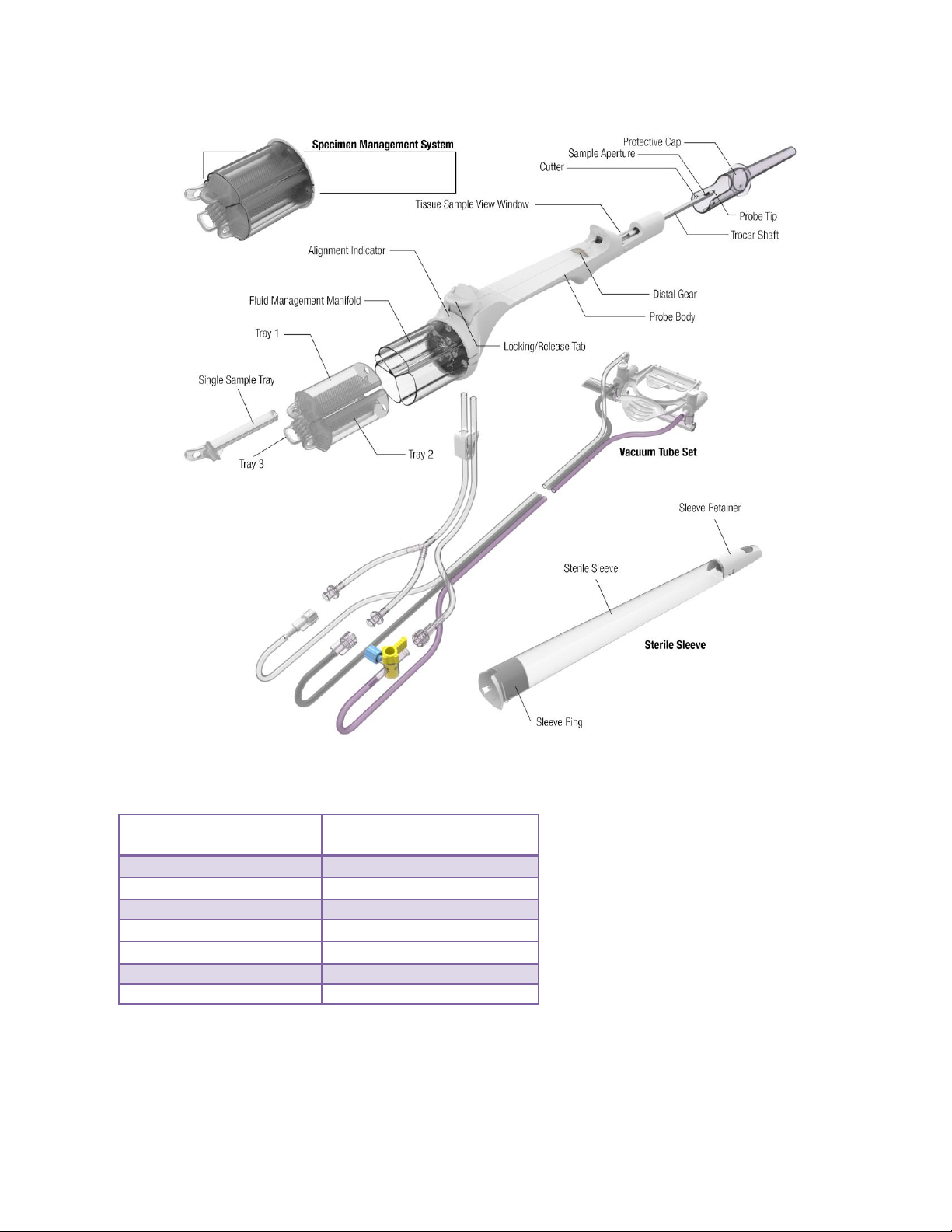
2-10
Figure 10. EX Probe, Vacuum Tube Set, and Sleeve Illustrations and Nomenclature
Table 3. Mammotome revolve EX Probe Specifications
Dimension (mm)
8G
MHEX08 AND MHEX08S
A: Needle Height
5.99 mm (6.0)
B: Needle Width
4.26 mm (4.3)
C: Tip to Center
23.3 mm
D. Needle Length
92 mm
E: Dead Space
10.8 mm
F: Aperture Length
25.0 mm
Fill Volume (cc)
9
Table of contents
Other mammotome Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual