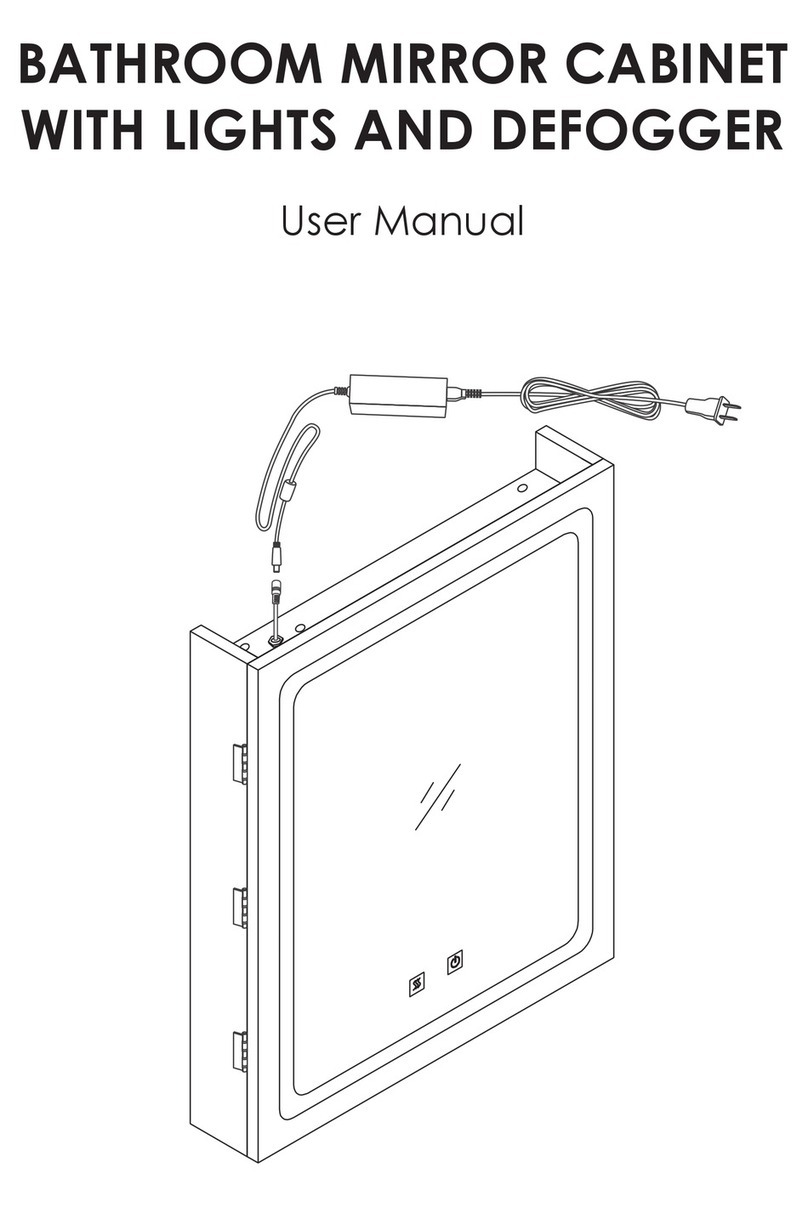
Neurowave PrimaBella WB-DL User manual

DESCRIPTION: PrimaBella is a transdermal neuromodulation device that generates
uniquely programmed pulses to stimulate the median nerve on the underside of the wrist.
These pulses (specific in waveform, frequency and intensity) create electrical signals that
travel to the central nervous system and the higher emetic center in the brain. These
signals act to positively modulate neural pathways—via the vagus nerve—thus restoring
normal gastric rhythm. PrimaBella is indicated for the treatment of nausea and vomiting
due to pregnancy (NVP) or morning sickness. PrimaBella will last approximately 100
hours when used on setting III.
™
WARNINGS:
• PrimaBella™should only be used on the designated area.
•Do notuse PrimaBella™when the cause ofnausea and vomiting symptoms are undiagnosed.
• Nausea and vomiting are serious medical conditions; seek medical attention if symptoms
continue.
•PrimaBella™is not a curative andshould always be usedunder medical supervision.
Treatment outcomemay vary dependingon patient characteristics and use of medications.
INTENDED USES: PrimaBella™is an FDA cleared device that is indicated for use in the
treatment of nausea and vomiting due to pregnancy (NVP) or morning sickness.
PrimaBella is for single patient use.
IMPORTANT: Adjust PrimaBella™and position on the wrist area until stimulation is felt.
You will feel a tingling sensation on the palm and/or middle when PrimaBella™is
in the proper wrist location. Beginning at the lowest setting (I), increase power level until
stimulation is consistently felt. Stimulation must be felt in the palm and/or middle
for PrimaBella™to provide relief.
CAUTION: This product contains natural rubber latex, which may cause allergic
reactions. Federal Law (USA) restricts this device to sale by, or by the order of,
CONTENTS: (1) PrimaBella™Neuromodulation Medical Device Model WB-DL(D),
(1)Hypo-allergenic conductivity gel tube, and Instructions for Use.
HOW TO USE: QUESTIONS AND ANSWERS
How can I be sure I’ve found the area for maximum stimulation?
• After the device is turned on, move it slightly up or down, and side to side on the wrist
until the maximum “tingling” feeling is felt. You will feel a tingling sensation in your palm
and/or middle when PrimaBella™is in the proper wrist location. Stimulation will
cycle every four seconds.
• If little or no tingling is felt, increase the power level. This device has levels of stimu-
lation: I, II, III, IV and V(level Vis the highest setting). If you are at level Vand do not
feel stimulation, curl your slightly and turn the hand inward (thumb towards your
body). If the stimulation increases, tighten the band at that point.
• Try the band on the opposite wrist. Use it on the wrist where you feel the most consistent
tingling. Increase or decrease power setting until stimulation is consistently felt.
• Re-apply gel as directed.
On which wrist should I wear PrimaBella™?
• On the wrist that gives you the greatest tingling feeling at the lowest stimulation level.
How do I turn off PrimaBella™?
• Press and hold down the power button ( ) for three (3) seconds.
Is dry or sensitive skin a problem?
• For dry skin, the gel may be lightly applied more often.
• If you have especially sensitive skin, switch wrists every 2-3 hours. Be sure to re-apply
gel as directed.
How can I be sure PrimaBella™stays in place at night?
• It may help to secure PrimaBella™in place by using strips of medical tape or an ace
bandage to secure it over the area while you sleep.
How long will the batteries last?
•
• Check electrode area for gel and clean as needed. Only apply enough gel to achieve a
sheen on the skin. Wipe gel from skin and device after each use.
LIMITED WARRANTY STATEMENT - PrimaBella™Medical Device Model No. WB-DL(D)
Neurowave Medical Technologies warrants each new PrimaBella™Neuromodulation Medical Device Model No.
WB-DL(D) to be free from defect in material and workmanship for approximately 100 hours of continuous
or intermittent use exclusive of batteries. All other warranties, express and implied, are limited to 60 days
from the date of purchase.
PrimaBella™is intended for use in the treatment of nausea and vomiting (NV) due to pregnancy. PrimaBella™
is not warranted to be effective in every case since treatment outcome varies dependent upon patient
characteristics.
The obligation of Neurowave Medical Technologies is expressly limited solely and exclusively to the replace-
ment of the unit to which Neurowave Medical Technologies’ satisfaction is defective. Contact Neurowave
Medical Technologies at 1-877-735-2263 for repair or replacement within the warranty period. Some States
do not allow limitations on how long an implied warranty lasts, so the above limitation may not apply to you.
This warranty does not extend to any liability for medical or dental expenses or for any other direct, indirect
or consequential damages caused by failure, defect or malfunction of any PrimaBella™except as herein
provided, whether such damage claim shall be based on contract, tort, breach or warrant, or otherwise. Some
States do not allow the exclusion or limitation of incidental or consequential damages, so the above limitation
may not apply to you.
This warranty shall not apply to any PrimaBella™which has been repaired, tampered with or altered by some-
one other than a duly authorized Neurowave Medical Technologies representative, nor to any PrimaBella™
which has been subjected to negligence, accident, mishandling or which has not been used in accordance
with the enclosed instructions or for the stated purposes.
This warranty is expressly limited solely to the original purchaser (consumer) and does not extend to any
transferee, assignee, or subsequent purchaser or user of any PrimaBella™. Neurowave Medical Technologies’
liability for all claims (whether based on contract, tort, breach of warranty, or otherwise) which may arise in
connection with the purchase and use of any PrimaBella™is limited to the purchase price paid by the original
purchaser therefore.
This warranty gives you legal rights, and you may also have other rights which vary from state to
state.
Rx Only
TECHNICAL DATA
Size .........................................Teardrop shape 1.5” x 2” x 0.45” (3.81 x 5.08 x 1.14 cm)
Weight.....................................Approximately 1.2 ounces (34 grams)
User Controls..........................Push Button
On/Power Level.......................Flashing green light indicator
Low Battery.............................Flashing red light indicator
Output Channels .....................Two electrodes
Maximum Output ....................40mA (nominal)
Battery ....................................One 3V lithium coin cell (CR2025) and
One 3V lithium coin cell (CR2016) pre-installed
1. Find the correct area
3. Attach to wrist
2. Apply gel
4. Activate
• Pacemaker users – Use this device only as directed on the wrist to prevent possible
interference with your pacemaker. Avoid placing the electrodes directly on your chest or
near the pacemaker. Consult with your physician if you have any other implanted devices.
• PrimaBella™should not be used above an IV line attached to a patient’s arm. If a patient is
using an IV line, PrimaBella™should be placed on the opposite arm.
SIDE EFFECTS:
• Isolated cases of skin irritation may occur where the electrodes touch the skin following
long term application. Continued use of the device on irritated skin may cause injury.
• If local skin irritation (redness, swollen, blotchy or itching on the wrist under the device)
occurs, discontinue use. If irritation does not disappear within 24 hours, consult your
doctor or pharmacist.
MAINTENANCE: Keep the electrode area clean. A damp cloth or alcohol wipe may be used.
Do not put the device in water.
LOW BATTERY WARNING: Low battery indicator when the batteries are
low.
(Approximately 10 hours of use remain depending on the power level used.)
ENVIRONMENTAL USE INFORMATION:
PrimaBella™should only be used within the environmental
ranges of: 10°C to 45°C (50°F to 113°F) and 20% to 90% rela-
tive humidity (non-condensing).
ENVIRONMENTAL STORAGE INFORMATION:
The device can be stored and transported in the environmental
ranges of: 0°C to 50°C (32°F to 122°F) and 20% to 90% relative
humidity (non-condensing). If the device has been stored or
transported in conditions outside this range, keep it within the
normal ranges for at least 30 minutes before using.
CAUTION: The batteries used in this device may explode if mistreated. Do not
recharge, disassemble, or dispose of in
The device and batteries do not contain any environmentally hazardous substances and can
be disposed of normally following your local laws.
PrimaBella™is not recommended for use in conjunction withelectrocautery or MRI equipment.
FCC INFORMATION: This device complies with Part 15 of the FCC Rules. Operation is
subject to the following two conditions, (1) This device may not cause harmful interfer-
ence, and (2) this device must accept any interference received, including interference that
may cause undesired operation.
SAFETY INFORMATION: PrimaBella™complies with IEC-601-1 (1988) Medical Electrical
Equipment, Part 1: General Requirements for Safety, including Am. No. 1 (1991) andAm. No
2 (1995) and EN 60601-1. PrimaBella™has been as a Class B Digital device per FCC
47 CFR, Part 15 Subpart Band has beenfound to comply with the following Electromagnetic
Compatibility standards: EM55011 (CISPR 11).
Radiated Emission: EN61000-4-2 Electrostatic Discharge Immunity; ENV 50204 and EN
61000-4-3 Radiated Electromagnetic Field Immunity.
PrimaBella™is
as TYPE B equipment.
PrimaBella™is
splash resistant.
Attention, PrimaBella™produces physiological
effects as described in this guide.
California Only: Perchlorate Material –
Special handling may apply. See
www.dtsc.ca.gov/hazardouswaste/perchlorate.
IPX4
Not replaceable, not rechargeable
200 East Randolph Street
Chicago, IL 60601 USA
PrimaBella™ is a trademark
of Neurowave Medical Technologies.
US Patent No. 6,735,480
Canadian Patent No. 1,319,174
European Patent No. 05000552
Manufactured in Malaysia
©2011 Neurowave Medical Technologies.
In the U.S.A., call 1-877-735-2263
6003840 Rev. X3
Manufactured for
™
™
™
a licensed health care provider with prescribing authority in the state in which they practice.
The batteries should last approximately 100 hours when used on power level III. Low
battery indicator flashes when the batteries are low. Actual consumption depends on
individual usage of the product. Dispose of the device when batteries are depleted.

Lacing the Elastic Band
Through the Clasp:
Re-apply Gel:
PrimaBella™Instructions
Read All Instructions First
Use eitherwrist. Looking at your palm, bend your wrist
slightly toward you (your should be pointing
toward your face). The correctarea is in between thetwo
tendons on the underside of your wrist, about 2
widths from the hand/wristcrease (see Figure 1).
Find the Correct Area:
1
After the correct area, clean as needed. Apply
the gel and spread to a thin sheen about the size of a
quarter or half-dollar. Do not use too much or too little
gel (see Figure 2). Too much gel may reduce intensity of
stimulation.
Apply Gel:
2
The wristband comes already laced through the metal
clasp. Just loosen enough to slip over your hand onto
your wrist. Place PrimaBella™over the gelled area and
then adjust the wristband by pulling the elastic end
until an appropriately tight is reached. The should
be snug enough not to move around on your wrist but
not so tight that it could affect blood to your hand.
Attach to Wrist:
3
If you pull the wristband all the way out of the metal
clasp, follow these steps to re-lace it.
1. First insert the end of the wristband through the
middle slot of the metal clasp (see Figure 5 and 7).
2. Then, feed it back through the open end slot of the
clasp (see Figure 6 and 7).
3. Any additional wristband can be tucked under the
keeper (belt loop).
The elastic wristband is designed to most wrist
sizes. Simply adjust the wristband by pulling the end
through the metal clasp until an appropriately tight
is reached. The should be snug enough to not move
around on your wrist but not so tight that it could affect
blood to your hand.
Turn PrimaBella™on by pressing the power button
( ). The blinking green light shows the power setting.
Beginning at the lowest setting (I), increase the power
setting by pressing the power button until stimulation is
consistently felt (see Figure 3 below and Figure 4 at the
top of the page).
Activate:
4
5
I
II
III
IV
V
LOW BATTERY
INDICATOR
ELASTIC
WRISTBAND
POWER BUTTON
Press to turn ON
Press again to increase
to the next level
Press and hold for 3 to 5
seconds to turn OFF
POWER LEVELS
(I- V)
ELASTIC
KEEPER
(BELT LOOP)
Features and Components
Figure 1
Figure 4
Figure 2
Figure 5 Figure 6
Figure 7
Figure 3
Re-apply gel as needed (see Figure 2) to maintain the
best quality of sensation (about every 2-3 hours).
Note: If there are blobs of gel, PrimaBella™may not
provide enough tingling. If you cannot feel the tingle,
clean your wrist and the back of PrimaBella™, then
re-apply the gel. You can apply extra gel if the tingle
feels too strong on level I.
ELASTIC
WRISTBAND
MIDDLE SLOT
END SLOT METAL
CLASP
This manual suits for next models
1
Popular Personal Care Product manuals by other brands

TrueLife
TrueLife NannyTone VM3 instructions

drybar
drybar The Single Shot 900-2840-4 Operating instructions & safety guide

Silk'n
Silk'n FaceTite H2111 user manual
nuskin
nuskin ageLOC LumiSpa iO Faq

Orliman
Orliman THERMOMED SMART 4604 Use and maintenance instructions

Coline
Coline PSA-9003 quick start guide