NIHON SEIMITSU SOKKI NISSEI DS-11 User manual

DS-11/DS-11a
JAPAN
UKRKAZ RUSUZB ENG
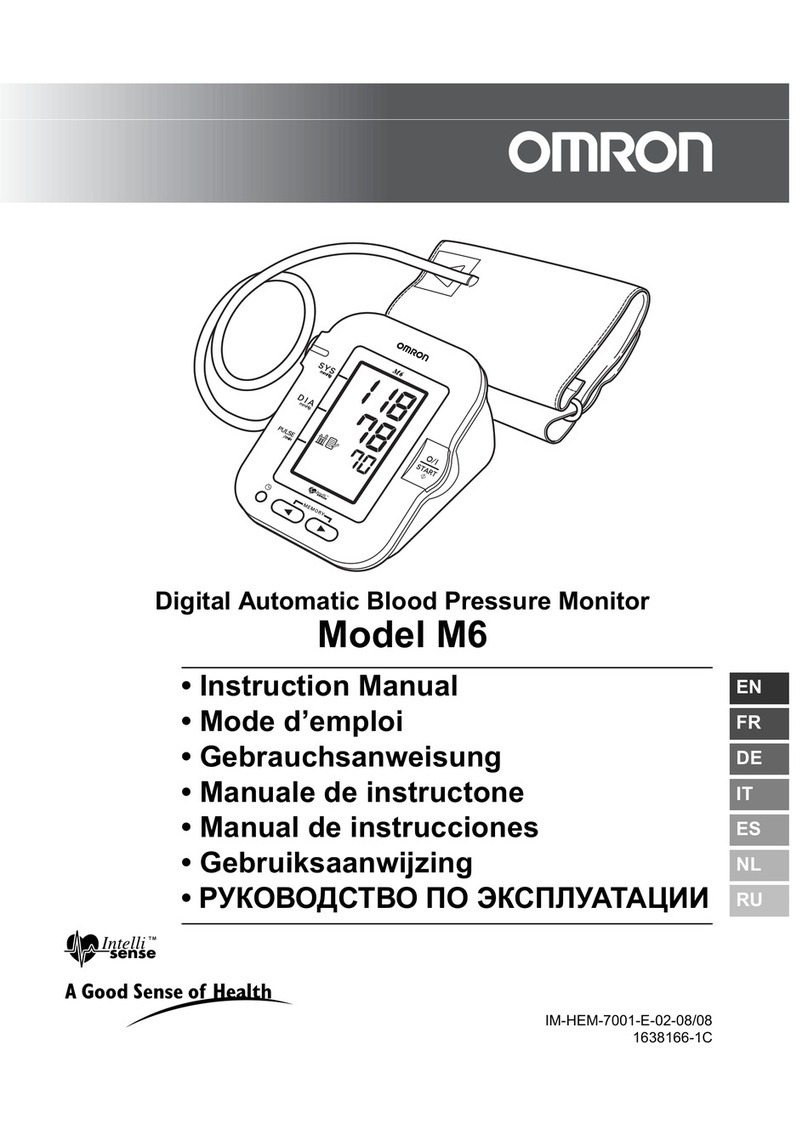
Digital blood pressure monitor DS-11, DS-11a
Instructoin manual
Kүретамырдың қан қысымы мен тамырдың соғу жиілігін
өлшеуге арналған сандық DS-11, DS-11a аспабы
Пайдалану жөніндегі басшылық құжат
Артериал босим ва пульс частотасини ўлчаш асбоби,
рақамли DS-11, DS-11a ижроси
Фойдаланиш бўйича қўлланма
Прибор для измерения артериального давления
и частоты пульса цифровой, исполнения DS-11, DS-11a
Руководство по эксплуатации
Вимірювач артеріального тиску та частоти серцевих скорочень
DS-11, DS-11a (Digital blood pressure monitor DS-11, DS-11a)
Інструкція з експлуатації

2
ENG
PARTS NAME AND PRODUCT COMPONENTS
3
4
5
5
1. MAIN UNIT
2. CUFF
3. BAG
4. AA (LR6) BATTERIES
5. AC ADAPTOR (only for DS-11A,
not included in DS-11)
a. LCD-display
b.
« »
START/STOP BUTTON
c.
«
M
»
MEMORY BUTTON
d. AC ADAPTOR JACK
e. AIR CONNECTOR
f. BATTERY COMPARTMENT
g. METAL RING
h. AIR HOSE
i. AIR PLUG

3
ENG
DISPLAYS
1. PULSE RATE MARK
2. MEMORY BANK SYMBOL
3. BATTERY REPLACEMENT INDICATION
4. BODY MOTION SYMBOL
5. IRREGULAR PULSE RHYTHM SYMBOL
6. INFLATION SYMBOL
7. DEFLATION SYMBOL
8. SYSTOLIC
9. DIASTOLIC
10.PULSE RATE
11. MEMORY NUMBER OR DATE/ TIME
GENERAL INFORMATION
This manual is intended to assist you in the safe and efficient operation of BLOOD PRESSURE
MONITOR DS-11 (DS-11A). The product must be used in accordance with the procedures contained in
this manual and must not be used for purposes other than those described herein. It is important to
read and understand the entire manual. In particular, please read carefully and become familiar with
the section entitled “TIPS ON TAKING YOUR BLOOD PRESSURE”.
INDICATIONS FOR USE
This product is intended for noninvasive measurement of systolic and diastolic blood pressure and
determination of pulse rate in adults in a home healthcare environment. The product is not designed
for neonatal use. Please consult with your doctor or physician to use this product to take blood pressure
of child or person in pregnancy or under pre-eclamptic condition.
METHOD OF MEASUREMENT
This product employs the oscillometric method for measurement of blood pressure and pulse rate.
The cuff is connected to the main unit and wrapped around the arm. When the
« »
button
is pressed, the device starts to pump automatically, during which the blood pressure is measured.
Circuits within the cuff sense the small oscillations in pressure against the cuff produced by the
expansion and contraction of the arteries in the arm in response to each heart beat. The amplitude of

4
ENG
each pressure waves is measured, converted to millimeters of mercury, and displayed on the LCD as
a digital value.
NISSEI New Technologies
Measurement upon inflation (Measurement on inflation) – is a technology that makes it
possible to define the pressure in the course of the cuff inflation.
Irregular Pulse Rhythm indicator – is a special icon on the display that informs on the
irregular heartbeat, while the measurement result is correct.
Body motion indicator - indicator informs the occurrence of possible body motion that
can affect the measurement result.
COMPLETE SET
The complete set DS-11 (DS-11a) includes:
- Electronic unit – 1 pc.
- Cuff model Cuff DS-11 (including Air hose and Air plug) – 1 pc.
- Batteries – 4 pcs.
- AC Adaptor ADP-W5 (for DS-11a only) – 1 pc.
- Bag – 1 pc.
- Instruction Manual – 1 pc.
- Warranty – 1 pc.
- Packaging – 1 pc.
RECOMMENDATIONS ON CORRECT MEASUREMENTS
1. If treated with hemodialysis or anticoagulants, antiplatelets or steroids, refer to your doctor about
the blood pressure measurement.
2. Malfunctions are possible when the device is used near working mobile phones, microwave ovens
and other equipment generating electromagnetic radiation.
3. For correct measurement it is necessary to know that the BLOOD PRESSURE IS SUBJECT TO SHARP
FLUCTUATIONS EVEN IN SHORT TIME INTERVALS. The blood pressure level depends on many factors.
It is commonly lower in summer and higher in winter. Blood pressure varies along with atmospheric
pressure and depends on the physical exertion, emotional excitability, stress and diet. Medical drugs,
alcohol and smoking exert great influence as well. Occasionally, measurements in the clinic cause an
increase in pressure values. Therefore, blood pressure measured at home is often different from that
measured in the clinic. Since blood pressure increases at low temperatures, measurements should be
made at room temperature (about 20°C). If the device was stored at low or high temperature outside
the operational temperature range prior to using, it should be kept for at least 2 hours at room tem-
perature. Otherwise the measurement result can be erroneous. During the day, the difference in the
readings in healthy people may attain 30-50 mm Hg for systolic (upper) pressure and up to 10 mm Hg
for diastolic (lower) pressure. Dependence of blood pressure on various factors is individual for each

5
ENG
person. Therefore it is recommended to keep a special recording of blood pressure readings. ONLY A
DOCTOR MAY ANALYZE TRENDS IN CHANGING YOUR BLOOD PRESSURE BASED ON CORRESPONDING
RECORDINGS.
4 In case of cardiovascular diseases and a number of other diseases that require the blood pres-
sure monitoring, measurements should be carried out in the hours specified by a doctor. REMEMBER
THAT THE DIAGNOSTICS AND ANY TREATMENT OF ARTERIAL HYPERTENSION SHOULD BE CARRIED
OUT ONLY BY A DOCTOR BASED ON BLOOD PRESSURE READINGS OBTAINED BY A DOCTOR. MEDICAL
DRUG ADMINISTRATION OR CHANGE OF DOSAGES SHOULD BE MADE ONLY BY PRESCRIPTION OF AN
ATTENDING DOCTOR.
Blood pressure variations during a day
Systolic
Diastolic
Arterial pressure (mmHg)
Time of day
Fig.1
5 In case of disorders such as deep vascular sclerosis, weak pulse wave and break in rhythm of heart
contractions, the correct blood pressure measurement can be complicated. IN THIS CASE, A DOCTOR
SHALL PROVIDE RECOMMENDATIONS IN RELATION TO USE OF THIS DEVICE.
6 KEEP QUIET DURING THE MEASUREMENT TO OBTAIN THE CORRECT BLOOD PRESSURE READING WHEN
USING THE ELECTRONIC DEVICE. The blood pressure measurement should be carried out in a quiet
comfortable atmosphere at room temperature. Exclude meal an hour before the measurement, and exclude
smoking, soft drinks, and alcohol 1.5-2 hours before the measurement.
7 Accuracy of the blood pressure measurement depends on matching the device cuff and size of
your arm. THE CUFF SHOULD NOT BE TOO SMALL OR TOO BIG.
8
Repeated measurements are carried out at 5-minute intervals to recover the blood circulation.
However, persons suffering from severe atherosclerosis, due to a significant loss of elasticity of
blood vessels, need longer intervals between measurements (10-15 minutes).
This also concerns patients suffering from long-term diabetes. For more accurate determination of
blood pressure it is recommended to carry out a series of three consecutive measurements and to
calculate the average value of measurement results.
9 Do not use this device in an explosive environment such as near flammable anesthetics or inside
oxygen chamber.
10 The system may fail to yield specified measurement accuracy if operated or stored in temperature
or humidity conditions outside the limits stated in the specifications section of this manual.

6
ENG
11 Do not use cuffs or accessories other than those specified by the manufacturer. Otherwise, correct
measurement readings cannot be obtained.
12 Do not apply the cuff over wounded arm, arm under an intravascular access or therapy or an
arterio-venous shunt, or arm on the side of a mastectomy or lymph node clearance. Otherwise injury
may be resulted.
13 Make sure that inflation of the cuff is not causing prolonged impairment of blood circulation. Also,
be cautious about temporary loss of the functions of any other medical equipment if any monitoring
equipment is used on the same limb with the blood pressure measuring cuff.
14 To avoid harmful injury due to interfered blood flow from cuff inflation, make sure that AIR HOSE is not
kinking before measurement. Otherwise, cuff inflation may not be conducted properly and prolonged.
15 Do not take out batteries or unplug the AC adaptor when the device is turned on. Make sure to
switch off the device before removing batteries or AC adaptor.
16 Do not touch the output plug of AC adaptor during measurement.
17 Do not inflate the cuff when it is not wrapped around your arm.
18 Do not apply the cuff on the limb which the intravenous drip infusion is implemented.
POWER SUPPLY OF THE DEVICE
Fig.2
1. 2.
3. 4.
1.
Open the battery compartment (fig.2.1).
2. Install four“AA” batteries in the compartment.
Make sure that polarity corresponds to signs (+) and (-) shown
inside the compartment (fig
.2.2).
Batteries are readily installed by pressing the end “-“ on the spring.
3. Close the battery compartment. Do not use excessive force
when removing the cover (
fig
.2.3).
Do not use excessive force when removing the cover
.
Battery Replacement Indicator
Replace all the batteries when
the battery
replacement indicator is
flashing on the display during the
measurement. If upon the device turning
on the indicator is steadily flashing, the
measurement will not be possible until
all the batteries are replaced. The battery
replacement indicator does not show a
discharge degree.
Use alkaline batteries to increase the de-
vice operation duration. Ordinary zinc-
carbon batteries require more frequent
replacement. The enclosed batteries are
meant for testing the sold device, and their
operation period can be less than that of
batteries acquired in the trade network.
Since neither the device nor the
batteries are the waste that can be
utilized at home
, follow your national/local
regulations for waste recycling and take
them to corresponding collection facilities.

7
ENG
USE OF THE DEVICE WITH THE AC ADAPTOR
Socket for the AC Adaptor is arranged on the right side of the
device (fig.2.3)
To use the device with the AC Adaptor, connect it to the de-
vice, install the power plug of AC Adaptor into the socket out-
let, and press the « » button.
When finished, turn off the device by pressing
the
« »
, button, unplug the
AC Adaptor
from the
socket outlet and disconnect it from the device.
NOTE!
If there is no battery in the device,
turning off the power source will
result in zeroing of measurement
results stored in the device memory
and set date and time. If you do not
want to make the data erased, do not
remove the batteries from the device
when using the power source.
ACTIVATION AND SETTING THE DATE AND TIME
Initially, the date/time function is not activated in the device.
Activating/deactivating the date/time function
1. Remove the batteries from the device.
2. While holding the « » button pressed, set the batteries.
3. When all the characters appear on the screen, release the « »button and press the“M”button.
If the date/time function is activated, then the time will be displayed when the device is turned off.
Setting the date/time
After replacing the batteries or after activating the date/time function, the date and time should be set.
The data is displayed as a four-digit number on the screen.
Set the year by pressing the “M” button. Confirm the selected year by pressing the « » but-
ton. The device will proceed to setting the date.
Press the “M” button to set the month and confirm the entry with the « » button. The de-
vice will proceed to setting the time.
Press the “M” button to set the hours and minutes and confirm the entry with the « » button.
Changing the date/time
Take out a battery, and put it back after the display goes blank. And then activate the date/time
function and reset the date and time again.
CORRECT POSITION DURING MEASUREMENT
Sit down at the table with your back supported
and feet flat on the floor so that during the blood
pressure measurement your forearm and hand
are on its surface.
Make sure that the place
where the cuff is put on the upper arm is about
the same level as the heart and the forearm
and hand freely lie on the table and does not
move (fig. 3).
Fig.3 Fig.4 Fig.5

8
ENG
You can also measure your blood pressure when lying on your back. Look up, stay calm and do not
move during the measurement. Make sure that the place where the cuff is put on the upper arm is
about the same level as the heart (fig. 5).
Measured values may vary slightly, depending on the position during the measurement. If the cuff is
above/below the level of the heart, resulting reading may be incorrect (lower/higher)
.
CUFF PREPARATION
Fig.6
1 Apply the cuff to your left upper arm so that the air hose is directed to
your palm (fig.6). If the measurement on your left arm is difficult, you may
use your right arm. In this case remember that the readings may differ by
5-10 mmHg and even more.
Fig.7
2
Wrap the cuff around your upper arm so that the bottom of the cuff is
approximately 2-3 cm above your elbow. Air tube should be directed towards
the palm
(fig.7)
.
Fig.8
3 Fix the cuff so that it fits tightly to the arm, but see that it is not overtight
(fig. 8). Too tight or too free placement of the cuff may give inaccurate
readings.
Fig.9
4 If the arm is cone-shaped, it is recommended to put the cuff spirally, as
shown in the figure (fig.9).
Fig.10
5 If the rolled-up sleeve squeezes the arm interfering with free blood flow
the Device may give inaccurate figures not corresponding to your actual
blood pressure (fig.10).

9
ENG
MEASUREMENT PROCEDURE
1. Insert the Air Plug into the Air connector
(fig.11)
.
Do not move, do not speak and do not toughen your arm.
2. Press « » button. After the full display appears and disappears,
deflation mark flashes and the calibration is adjusted to the ambient
air pressure (fig.12).
Automatic inflation starts and inflation mark
flashes.
3. Automatic inflation starts and inflation mark « » flashes (
fig
.13).
4. IInflation mark disappears and measurement begins.
Noise Interference Detection
Blood pressure value taken while moving cannot be said to be the
correct value because body movement can affect blood pressure.
This product analyzes pulse wave and displays « » when body motion
is detected. Symbol « » indicates the results might be affected by body
movement.
Press the «»button to stop forcedly the measurement: the de-
vice will stop inflation and quickly release the air.
5. Heart mark flashes as pulse is detected «» (fig. 14).
The unit automatically exhausts the air from the cuff as the
measurement is complete.
6. Blood pressures and pulse rate are displayed
(fig. 15)
.
The reading is automatically saved in the bank.
7. Press the
« »
START/STOP button to turn off the device.
If you forget to turn off the device, it will do so automatically after
3 minutes.
Do not perform several measurements in a row.
This will cause numbing the arm and can affect the measurement
result. Give your arm a break for at least 5 minutes.
Fig.13
Fig.14
Fig.15
Fig.12
Fig.11

10
ENG
IRREGULAR PULSE RHYTHM INDICATION
Pulse rhythm can be disturbed from talking, moving or arrhythmi-
as. This product displays « », indicating irregular pulse. (fig.16).
Although continuous appearance of the indication under quiet
measurements may suggest arrhythmias, do not make any judg-
ment on your own before consulting with your doctor.
MEMORY FUNCTION
The measured values are automatically saved in memory for later view-
ing. The device can store up to 60 measurement results and their aver-
age value. When the number of the values stored exceeds 60, the oldest
measurements is deleted in order to record new values.
View the saved data
1. Turn off the device by pressing the « » button.
2. To view the saved results, press the “M” button. The average for up
to 3 readings before the last measurement is displayed with «A3»
symbol. Average will not be displayed unless there are two or more
readings saved.
3. Each time you press the “M” button, the stored measurement re-
sults will be displayed successively.
4. In the lower left corner of the display, the memory cell number,
date and time of measurement will be displayed interchangeably
(Figure 17). The result stored in the cell at number 1 is the most re-
cent among the stored data in the selected memory. The larger the
memory cell number is, the older is the result.
5. Press the « » button to turn off the device.
Deleting the saved data
1. Select a value from the memory block that you want to delete, or
an average value to delete all data from the memory.
2. Press and hold the “M” button (Memory). The displayed data will
start to flash.
3. Press the button until the stored data disappears.
Normal pulse
Pulse with arrhythmia
Fig.16
Fig.17

11
ENG
INFORMATION ABOUT ERRORS
INDICATION LIKELY CAUSE
METHODS OF CORRECTION
Blood pressure is
extremely high or low.
The cuff is not at the heart level.
The cuff is put on incorrectly.
During the measurement, a person was
talking or moving.
Put on the cuff at the heart level.
Check the cuff position on the arm.
Be calm and quiet during the measurement.
Measurement results are
different each time.
Effect of measurement conditions, physical
or mental state.
Take measurements under the same conditions
Measurement results are
different in clinic and
at home.
Effect of relaxed state at home and tension
in clinic.
Show the pressure records made at home to
your doctor for advice.
Предельно допустимое давление:
давление не может быть измерено из-
за движения или разговора во время
измерения, хотя манжета нагнеталась
максимально.
Во время измерения не разговаривайте и не
двигайтесь.
Pressure can not be measured due to
movement or talking.
Do not talk and do not move during the
measurement.
Cuff is not securely connected to the device.
Cuff is not put on properly.
Check the connection.
Make sure that the cuff is put on correctly.
Batteries are discharged. Replace all batteries with new ones.
No indication on the
display.
Batteries are discharged.
Batteries are installed incorrectly.
Connecting terminals are contaminated.
Power source is not connected.
Replace all batteries with new ones.
Install batteries properly.
Wipe connecting terminals with a dry cloth.
Connect the power source.
The time is not
displayed on the
screen
Clock function is disabled. When the battery and/or the power supply
unit are removed, the clock function is
disabled. Set the date and time, then
activate the clock function.

12
ENG
Date and time of
measurement are
displayed on the screen
as [-: -] and [-- / --].
Measurements were taken with the
clock function disabled. Date and time
of the measurements cannot be saved
without activating the clock function
Set the date and time, then activate the clock
function.
You pressed the
« »
button when
installing the batteries
.
Turn off the device by pressing the « »
button and perform the measurement again
If, despite the above-given recommendations, you fail to obtain the right measurements, stop the operation and
contact the service center (addresses and telephone numbers of authorized organizations are provided in the
warranty certificate). Do not attempt to adjust the device internal mechanism on your own.
WARRANTY
1. The manufacturer guarantees the warranty period of 5 years for the device from the sale date
provided that the consumer observes operation, transportation and storage requirements. The
warranty period for the cuff and the AC adpator is 12 months from the sale date.
2. Warranty liabilities are documented with the warranty certificate upon selling the device to the buyer.
The guarantee is valid provided that the device has not been opened or damaged by the buyer.
3. Addresses of organizations engaged in the warranty service are specified in the warranty
certificate.
TECHNICAL SPECIFICATIONS
Operating Principle Oscillometric method
Indicator 13-digit LCD display
Pressure Indicating Range 0 to 300 mmHg (cuff pressure)
Measuring Range:
cuff pressure
pulse rate
40 to 250 mmHg
40 to 180 mmHg
Accuracy:
cuff pressure
pulse rate
±3
±5
Cuff Cuff DS-11
Cuff size, cm 22-42
Operation conditions:
Temperature, °C
Relative humidity, % Rh
from 10 to 40
from 15 to 85

13
ENG
Storage and transportation conditions:
Temperature, °C
Relative humidity, % Rh from -20 to 60
from 10 to 85
Power Supply ,
V6
Inflation
automatic (air pump, Measurement on inflation)
Deflation automatic
Type of power supply
4“AA”size batteries (LR6) or adapter not less than 600 mA
ADAPTER ADP-W5 (included in DS-11A)
Output voltage, V
Max load current, А
Input voltage, В/Гц
6
0,5
100-240/50
Dimension, mm 120х125х70
Weight (without batteries and adapter), g 350
Year of manufacture: year the manufacture is given in the bottom of the Device
body in a serial number after symbols “AA”.
Protection class IP IP20: Protected against solid foreign particles with a
diameter of more than 12.5 mm, no protection against
water.
Protection against electric shock Internally powered equipment/Class II equipment, Type
BF applied part
Mode of operation Continuous operation
Classification Class II / Internally powered equipment
SYMBOLS:
Important: Read the instructions
Sign of type approval of measuring instruments
Type BF
Manufacturer
GOST conformity mark
Environmental Packaging
Protect from moisture
IP20
IP protection class
Class II
UA.TR.001
Mark of conformity of Ukraine
19
Sign of type approval of measuring instruments of Ukraine
When utilizing the waste, refer to current rules applicable in your
region
Compliance with Directive 93/42 / EEC
*This device complies with EN1060-1:1995+A2:2009 Non-invasive sphygmomanometers Part 1: General
requirements and EN1060-3:1997+A2:2009 Non-invasive sphygmomanometers Part 3: Supplementary
requirements for electro-mechanical blood pressure measuring system
*Accuracy is guaranteed with the measured values that are within the measuring range.
*The measurement accuracy of the device has been proven according to ISO 81060-2 protocol. In the clinical

14
ENG
study, K5 was used for the determination of diastolic pressure values at all auscultatory measurements.
*This device is intended for use in the environment with one atmospheric pressure.
*Specifications are subject to change without notice due to improvements in performance.
Revision date of the present Manual is indicated on the last page as IXXX/YYMM/NN, where YY is the year, MM
is the month and NN is the number of revision.
CARE, STORAGE, REPAIR AND DISPOSAL
1 This device should be protected from excessive moisture, extreme temperature variations, direct
sunlight, strokes, dust, lint and vibration. THE DEVICE IS NOT WATERPROOF!
2 Do not keep or do not use the device in close proximity to heaters and open flame.
3 In case the product is stored in the environment with ambient temperature above 40˚C or below
10˚C, please leave it for at least 2 hours before taking a measurement.
4 If the device has not been used for a long time, remove the batteries. Leaking of batteries can
cause damage to the device and terminate the warranty. KEEP BATTERIES AWAY FROM CHILDREN!
5 Do not contaminate the device and protect it from dust. The device can be cleaned with a dry,
soft cloth.
6 Do not allow the contact between the device and its parts with water, solvents, alcohol,
and gasoline.
7 Keep the cuff away from sharp objects, and do not try to pull out the cuff.
8 Do not expose the device to strong strokes and do not throw it.
9 The device does not contain any adjustment controls for settings. Unauthorized opening of the
electronic device is forbidden. If needed, repair the device only in specialized organizations.
10 On the expiry of the specified operation term, refer to specialists (specialized repair organizations)
on a periodic basis to check the technical condition of the device.
11 When utilizing the waste, refer to current rules applicable in your region. No special utilization
conditions are specified by the manufacturer for this device
12 Keep the device clean. Inspect its cleanliness after use. To clean, use only a soft dry cloth. Do not
use gasoline, paint thinner, or other strong solvents. The cuff is resistant to repeated sanitation.
The cuff internal fabric surface (being in contact with a patient’ arm) can be treated with a cotton
swab moistened in a 3% solution of hydrogen peroxide. Partial discoloration of the cuff covering
tissue is possible if used for a long time. Do not wash the cuff and do not treat it with a hot iron.
13 Do not leave unattended the device plugged into the network.
14 Stop using the device immediately and contact your dealer or the manufacturer in case any visible
damage is found on the device.
15To avoid any possibility of accidental strangulation, keep this device away from children and do
not drape AIR HOSE around your neck.
16 Do not press the display or place the device with display face down.
17 The device contains small parts and batteries which could be swallowed by children or pets. They
should therefore be kept out of the reach of children and pets at all times.

15
ENG
18 This device is not designed for self-use by unspecified persons in public areas.
19 Any serious incident occurred in relation to the device should be reported to the manufacturer and
the competent authority in your country/area. If you have no contact information of such authority,
please contact the manufacturer or EU authorized representative whose contact information is
indicated in this instruction manual.
CERTIFICATION AND STATE REGISTRATION
The production of devices is certified pursuant to international standards ISO 9001, ISO 13485, ISO 14001.
The device meets international standards IEC 60601-1:2005+A1:2012 and IEC 60601-1-2:2014.
AC Adaptor ADP-W5
meets international standard
IEC60601-1 by JQA, class II.
Produced by Nihon Seimitsu Sokki Co., Ltd.
Address: 2508-13 Nakago Shibukawa Gunma 377-0293 Japan
website: www.nissei.pl
EC-Representative: MDSS GmbH Schiffgraben 41, 30175 Hannover, Germany
TECHNICAL DESCRIPTION FOR ELECTROMAGNETIC DISTURBANCES
DS-11 (DS-11a) complies with the Electromagnetic Disturbances standard, IEC60601-1-2:2014.
As a medical electrical equipment, special precautions regarding the electromagnetic disturbances shall
be taken at usage of the device according to the information provided below.
• The device is not intended for use in environments where the intensity of electromagnetic disturbance
is high, such as near active HF surgical equipment and MRI (magnetic resonance imaging) equipment etc.
• Use of the device adjacent to or stacked with other equipment must be avoided because it could result
in improper operation.
• Use of accessories other than those specified or provided by the manufacturer could result in increased
electromagnetic emissions or decreased electromagnetic immunity of the device and result in improper
operation.
• Portable RF communications equipment (including peripherals such as antenna cables and external
antennas) should be used at least 30cm away from any part of the device, including specified cables.
Otherwise, degradation of the performance of this equipment could result.
Please contact your dealer or the manufacturer for specific information regarding the compliance to the
standard.

MDSS GmbH
Schiffgraben 41, 30175 Hannover, Germany
I570/1903/11
UA.TR.001
®Зарегистрированный товарный знак.
©Copyright 2019.
NIHON SEIMITSU SOKKI CO., LTD.
2508-13 Nakago Shibukawa Gunma 377-0293 Japan
web site: http://www.nissei-kk.co.jp/english/
19
This manual suits for next models
1
Table of contents
Other NIHON SEIMITSU SOKKI Blood Pressure Monitor manuals