Northern Meditec HF3 User manual
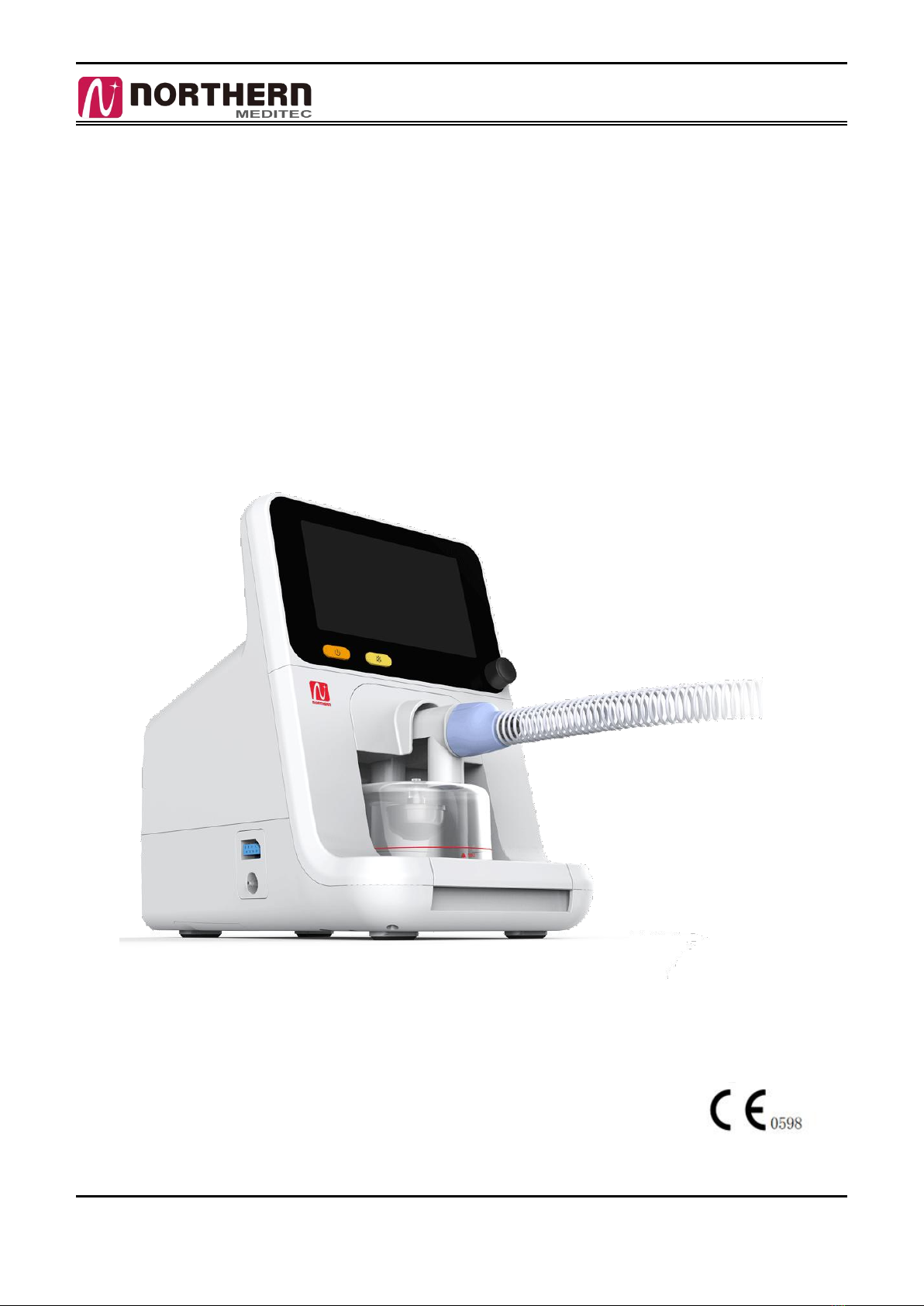
HF3 Operator’s Manual V1.1
High-flow nasal cannula Respiratory humidifier
HF3
Operator’s Manual

HF3 Operator’s Manual V1.1
Manufacturer and European Representative Information
MANUFACTURER:
Northern Meditec Limited
Room 501, 502 Building A, Room 401 Building C, JinWeiYuan Industrial Area, No.41 Qingsong Road,
Zhukeng Community, Longtian Sub-district, Pingshan District, Shenzhen City, China
Tel: +86 4000097972
Fax: +86-755-23010273
EU AUTHORISED REPRESENTATIVE:
Umedwings Netherlands B.V.health
Treubstraat 1,2288EG,Rijswijk, The Netherlands
Tel: +31(0) 642758955
E-mail:ar@umedwings.eu
File No.:NR-TP-0115-002 Version No:1.1 Issued Date:July 20, 2023

HF3 Operator’s Manual V1.1
page 3of 52
Table of Catalog
1. Symbols .................................................................................................................................................6
2. Warning, Caution and Important Tip .....................................................................................................7
3. Intended Use .........................................................................................................................................7
4. Contraindications .................................................................................................................................. 9
5. Specifications ...................................................................................................................................... 10
6. Available Therapies ............................................................................................................................ 13
7. Glossary .............................................................................................................................................. 13
8. Model ...................................................................................................................................................14
9. Package Contents ...............................................................................................................................14
10.System Features ................................................................................................................................16
11.Preparation .........................................................................................................................................18
11.1 Placement ................................................................................................................................18
11.2 Installation and Connection .....................................................................................................18
11.3 Oxygen Connection ................................................................................................................. 19
12.Use of Device .....................................................................................................................................20
12.1 Start-up the device .................................................................................................................. 20
12.2 User interface and parameter area ......................................................................................... 21
12.3 Parameter settings .................................................................................................................. 22
12.3.1 Main menu .....................................................................................................................22
12.3.2 Mode settings ................................................................................................................22
12.3.3 Temperature and humidity settings .............................................................................. 23
12.3.4 Flow settings ................................................................................................................. 24
12.3.5 Low flow mode .............................................................................................................. 25
12.3.6 Oxygen concentration settings ..................................................................................... 25
12.3.7 Review of trends ........................................................................................................... 25
12.3.8 Time setting ...................................................................................................................26
12.3.9 Lock screen settings ..................................................................................................... 27
12.3.10 System setup ...............................................................................................................27

HF3 Operator’s Manual V1.1
page 4of 52
12.3.11Event Logbook ............................................................................................................. 28
12.3.12 Warning ..................................................................................................................... 29
12.5 Battery Maintenance ................................................................................................................29
12.5.1Battery Performance Conditioning .................................................................................30
12.5.2 Battery Performance Checking .....................................................................................31
12.5.3 Battery Storage ............................................................................................................. 31
12.5.4 Battery Recycling .......................................................................................................... 32
13.Alarm .................................................................................................................................................. 32
13.1 Grading for Alarming and Description .....................................................................................33
13.2 Auditory Alarming .................................................................................................................... 33
14.Equipment maintenance and cleaning .............................................................................................. 36
14.1 Maintenance and care .............................................................................................................36
14.2 Time interval ............................................................................................................................ 37
14.3 Cleaning and disinfection ........................................................................................................ 38
14.3.1 Cleaning the Device ...................................................................................................... 39
14.3.2 Cleaning the Nasal Cannula ......................................................................................... 39
14.3.3 Cleaning the heating breating tube and water chamber .............................................. 39
14.3.4 Cleaning or Replacing the Air Filters ............................................................................ 39
14.3.5 Replacing the Nasal Cannula ....................................................................................... 40
14.3.6 Replacing the Tube and the Water Chamber ...............................................................40
14.3.7 Disinfection the Breathing gas pathways ......................................................................41
14.3.6 Reordering .....................................................................................................................41
14.4.1 Storage ..........................................................................................................................42
14.4.2 Transport ....................................................................................................................... 42
14.5.1 Main Unit Processing Instructions ................................................................................ 42
14.5.2 Notes on the handling of annexes ................................................................................ 43
15.Reordering ......................................................................................................................................... 43
16.Technical Support .............................................................................................................................. 43
17.Disposal ..............................................................................................................................................43

HF3 Operator’s Manual V1.1
page 5of 52
18.Trouble shooting ................................................................................................................................ 43
19. Working principle .............................................................................................................................. 45
20.Pneumatic Circuit Diagram ................................................................................................................45
21.EMC Requirements ............................................................................................................................46
22.Limited Warranty ................................................................................................................................52

HF3 Operator’s Manual V1.1
page 6of 52
1. Symbols
Symbols
Definition
Symbols
Definition
Power on/off Button
Mute Button
Follow Instructions for Use
Operating Instructions
Beware of hot hands
Cautions
Type BF application
components
Heating plate output
180W@220V
AC Power
DC Power
Serial Number of the Product
Lot number
Manufacturer
Catalogue Number
Use-by date
Date of Manufacture
≥ 12.5 mm Diameter, Dripping
(15º tilted)
WEEE Marking
Authorized Representative in
the European Community
European CE Declaration of
Conformity
Oxygen Inlet
Air Inlet

HF3 Operator’s Manual V1.1
page 7of 52
2. Warning, Caution and Important Tip
WARNING
Indicate the possibility of injury to the user or operator.
CAUTION
Indicate the possibility of damage to the device.
3. Intended Use
The HF3 High-flow nasal cannula Respiratory humidifier is intended to be used for the treatment of
spontaneously breathing adult and pediatric (greater than 5Kg) patients who are with type 1/ type 2 acute
respiratory failure with providing high flow warmed and humidified respiratory gases.The HF3 is for patients
in hospitals and long-term care facilities.
The product should be operated by properly-trained and healthcare professional operators.
This equipment is not suitable for use in an MRI environment.
This equipment is not intended for life support.
3.1 Structure and Composition
The high-flow nasal cannula Respiratory humidifier consists of a main unit,power cord,Li-ion battery,
support arm, and trolley.
Accessories (water chamber, heating breathing tube, nasal cannula) recommended to buy
EXCELLNETCARE MEDICAL (HUIZHOU) LTD.(Nasal Cannula model:EM05-501B,EM05-503B;Heated
Breathing Tube:LH2) or CE certified merchants.
WARNING
Do not bring the device or accessories into a Magnetic Resonance (MR) environment, it may cause
unacceptable risk to the patient or damage to the device or MR medical devices. The device and
accessories have not been evaluated for safety in an MR environment.
Do not use the device or accessories in an environment with electromagnetic equipment such as CT
scanners, Diathermy, RFID and electromagnetic security systems (metal detectors) as it may cause
unacceptable risk to the patient or damage to the device. Some electromagnetic sources may not be
apparent, if you notice any unexplained changes in the performance of this device, if it is making

HF3 Operator’s Manual V1.1
page 8of 52
unusual or harsh sounds, disconnect the power cord and discontinue use. Contact your home care
provider.
Several accessories are available to make your treatment with this device as convenient and
comfortable as possible. To ensure that you receive the safe, effective therapy prescribed for you, use
only Northern accessories.
Use-by date:Indicates the date after which the medical device is not to be used. If continue using the
product after the expiration date, it may cause harm to the patient or the operator.
Please do not insert an unauthorised device such as a USB or memory card into the HF3. This action
may damage the HF3 and cause the medical device to be unable to use.
Do not add any attachments or accessories to the equipment that contravene the instructions for
use of the equipment or accessory, as the equipment might not function correctly leading to the risk
of degradation of health of the patient.
Do not use sealed patient interfaces with this equipment, to avoid the risk of suffocation or
barotrauma.
There is a risk of fire associated with oxygen enrichment during oxygen therapy. Do not use the
equipment or accessories near sparks or open flames.
Use only water-based lotions or salves that are oxygen-compatible before and during oxygen
therapy. Never use petroleum-based or oil-based lotions or salves to avoid the risk of fire and
burns.
Do not lubricate fittings, connections, tubing, or other accessories of the equipment to avoid the
risk of fire and burns.
Use only spare parts recommended by the manufacturer to ensure proper function and to avoid the
risk of fire and burns.
If you wish to update the software or download the device using information, please conduct this action
under proper authorisation.
Note that if the patient's peak inspiratory demand exceeds the flow deliveredby the unit, the fraction of
oxygen inspired by the patient will be lower than thevalue shown onscreen, due to the additional
entrainment of ambient air.Check that suitable blood saturation levels are achieved at the prescribed
flow.
IMPORTANT TIPS!
Read and understand the entire user manual before operating this system. If you have any questions
concerning the use of this system, contact your home care provider or health care professional.
The pictures in the user manual are only for reference, if they are different from the material object, the
latter shall prevail.
Nasal delivery of respiratory gases generates flow-dependent positive airway pressure (PAP). This
must be taken into account where PAP could have adverse effects on a patient.
The user and/or patient shall report to the EU Rep and the manufacturer if there is any serious incident
that has occurred in relation to the device usage or other issues.
The unit is not intended for life support.
To avoid burns:
The unit should only be used with interfaces, water chambers and breathing tubes specified in this user
manual.
Using the breathing tube or interface for longer than the specified time can result in serious injury

HF3 Operator’s Manual V1.1
page 9of 52
including infection.
Before using oxygen with the unit read all warnings in the “Oxygen” section of this manual.
Never operate the unit if:
The heated breathing tube has been damaged with holes, tears or kinks.
It is not working properly.
The case screws have been loosened.
Do not block the flow of the air through the unit and breathing tube.
The unit should be located in a position where ventilation around the unit is not restricted.
Never block the air openings of the unit or place them on a soft surface such as a bed or couch/sofa,
where the filter area may be blocked. Keep the air openings free of lint, hair etc.
To avoid electric shock:
Do not store or use the unit where it can fall or be pulled into water. If water has entered the unit
enclosure, disconnect the power cord and discontinue use.
Never operate the unit if:
It has been dropped or damaged,
It has a damaged power cord or plug,
It has been dropped into water.
Avoid unnecessary removal of the power cord from the rear of the device. If removal is necessary, hold
the connector during removal. Avoid pulling on the power cord.
Return the unit to an authorized service centre for examination and repair, except as outlined in this
manual.
To avoid choking, or inhalation of a foreign object:
Ensure an air filter is fitted when operating four units.
Never drop or insert any object into any opening or tube.
Miscellaneous:
Do not use the unit when the room temperature exceeds 30℃(86℉)or is below 10℃(50℉)as the unit
may switch off. Humidity output will be compromised below 10℃(64℉) and above 28℃(82℉).
The unit is not suitable for use in the presence of a flammable, anaesthetic mixture with air or oxygen or
N2O
Smoking during oxygen therapy is dangerous and is likely to result in facial burns or death. Do not allow
smoking or open flames within the same room as the equipment or any oxygen-carrying accessories. If
the patient intends to smoke, always turn the equipment off, remove the cannula and leave the room
where the equipment is located. If unable to leave the room, wait 10 minutes after you have turned the
equipment off.
Ensure a sufficient intended leakage between the breathing system and the patient to allow the patient
to exhale.
4. Contraindications
Patients who meet the following conditions can use the device only under the special care of a physician
and monitoring on schedule.
Absolute Contraindications:

HF3 Operator’s Manual V1.1
page 10 of 52
Cardiopulmonary arrest, the invasive mechanical ventilation of an urgent trachea cannula is required
Shallow autonomous respiration and coma
Extremely severe Type I respiratory failure
Ventilation dysfunction (PH < 7.25)
Relative Contraindications:
Severe Type I respiratory failure
Ventilation dysfunction (PH < 7.30)
Paradoxical breathing
The protective capacity of the airway is poor, and there is a high risk of aspiration
The hemodynamics is unsteady, and the vasoactive drugs are required
The device cannot be worn in the facial or upper respiratory tract operation
The nasal cavity is seriously blocked
Intolerant of nasal humidifier
WARNING
Do not use the device if you suffer from severe respiratory failure without any autonomous respiration.
To avoid the risk of electric shock, this equipment must only be connected to a supply main with
protective earth.
CAUTION
Contact your healthcare professional if you have any questions concerning your therapy.
5. Specifications
5.1 Safety Specifications
Type of protection against electric shock
Class I equipment with internal electrical
power supply.
Degree of protection against electric shock
BF
Degree of protection against harmful ingress of water
IP21
Operating mode
Continuous
The degree to which flammable anaesthetic gases
mixed with air or with oxygen or with helium oxide are
safe to use
Do not use flammable anaesthetic gas
mixed with air or oxygen or flammable
anaesthetic gas mixed with helium oxide
5.2 Environmental Specifications
Parameter
Operation
Transport and Storage
Temperature
18℃~28℃
-10℃~+60℃

HF3 Operator’s Manual V1.1
page 11 of 52
Humidity
≤93%(Non-condensing)
10%~95%(Non-condensing)
Atmospheric Pressure
70~106kPa
50 to 106 kpa
High pressure oxygen access range
280kPa-600kPa
(43.50psi~87.02psi)
/
5.3 Power Requirements
External AC power supply
Input voltage
100-240V
Input frequency
50/60 Hz
Input current
2.5A-1.2A
Internal battery
Number of batteries
One
Battery type
Lithium-ion battery
Rated battery voltage
14.8 VDC
Battery capacity
5200 mAh
5.3 Physical Specifications
Sound Power Level
< 28 dB (A), When the working mode of the device is HFlow, and the
output flow is 25 L/min
Dimensions
L 310mm x W 228mm x H 322mm
Weight
Weight not more than 16 (Kg) (without peripherals)
Water capacity
To maximum fill line 235mL
5.4Performance Specifications
Parameter
Range
Accuary
Flow
2~25L/min,Step:1L/min
2L/min~20L/min: ±2L/min
21 L/min ~60 L/min: ±10%
10~60L/min,Step:1l/min
Temperature
HFlow mode:31℃,34℃,37℃
±2℃
LFlow mode:34℃
Oxygen concentration
21%~95%
±(2.5%+2.5% of gas level)
Oxygen sensor Specifications
Detection precision
±3%FS@(5~45)℃
Resolution
0.1%
Response time
<5s
Response Time 90%
T90 = 6 Seconds
Drift % Signal/Month
<1%
Temperature Coefficient
Compensated
Operating conditions
5~50℃;0~95%HR under
Average operating voltage
DC4.75-12.6V

HF3 Operator’s Manual V1.1
page 12 of 52
Mean operating current
<50mA
Alarm limit specification
Range
Step size
Notes
FiO2high alarm limit
19-100%
1%
Set the high alarm limit to be greater
than the low alarm limit.
FiO2low alarm limit
18-99%
1%
FiO2alarm limit setup:
Click on 【Menu】->Select [FiO2] interface, and set FiO2alarm high and lower alarm limit.
Other
Maximum oxygen input
≤80L/min
Maximum operating pressure
≤6kPa
Humidity
>33mg/L at 37 °C target
>10mg/L at 34 °C target
>10mg/L at 31 °C target
Warm-up time
10minutes to 31℃(88℉)
30minutes to 37℃(98.6℉)
Maximum temperature of
deliver gas
43℃(109℉)
Tube
Length: 1.8 m
Margin of Error: ±10%
The connection should not fall off under the 45 N tension.
Patient Connector
φ 22 mm cone joint
Uncertainty of measurement
Measurement Result:
Ue=1.23%,K=2,(level of confidence)P=95%
NOTE
Oxygen concentration monitoring does not provide automatic atmospheric pressure compensation. Do
oxygen concentration calibration again when atmospheric pressure has changed.
Increasing to periodical pressure of 10 kPa (100 cmH2O) has no effect upon oxygen concentration
monitoring accuracy.
O2 sensor measures the partial pressure of oxygen. Increase or decrease of pressure (absolute
pressure) affects the partial pressure of oxygen. Increase of pressure (absolute pressure) by 10%
causes oxygen concentration to increase by 10%. Decrease of pressure (absolute pressure) by 10%
causes oxygen concentration to decrease by10%.Do oxygen concentration calibration when
atmospheric pressure has changed.
Oxygen sensor calibration, automatic calibration, no operation.
Oxygen concentration measurement accuracy may be compromised due to:
Leakage or internal leakage of the sample gas.
Quantitative effects of gas sample humidity or condensate.
Other interference source (if available).

HF3 Operator’s Manual V1.1
page 13 of 52
6. Available Therapies
The device delivers the following therapies:
HFlow–Under this mode, the setting range of flow is 10L/min-60L/min.
LFlow–Under this mode, the setting range of flow is 2L/min-25L/min.
7. Glossary
Auto Off
When this feature is enabled, the device automatically stops outputting flow after the patient removes the
nasal cannula.
Delay
After pressing the stop button, the device stops outputting after continuing to work with the flow of not more
than 60 L/min for about 90min.
Standby
When this feature is enabled, the patient removes the nasal cannula, the FiO2drops to 21%, the flow drops
to below 40 L/min, it continues to heat, and the temp of the output gas of the patient does not exceed 43℃,
the maximum standby time is 30 min.
Warm-uptime
When the flow is 40 L/min and starting temperature is 23±2℃, the set temp of 29℃can be reached within
10 min, and the set temp of 37℃can be reached within 30min.
LCD Backlight
LCD Backlight can set the two modes. In backlight mode, the backlight is always on; in automatic mode, the
backlight can be dimmed for about 30 seconds without button operation, and brightened when there is a
button operation.
Trend Chart
The user can review the data of temperature, flow, FiO2 and respiratory rate for 1 day, 3 days, and 7 days.
LPM
Liters Per Minute.
min
Means the time unit ―minute.
h

HF3 Operator’s Manual V1.1
page 14 of 52
Means the time unit ―hour.
yy mmdd / mmddyy /dd mmyy
Denotes date.
8. Model
Model
Product information
Work Mode
Maximum flow(L/min)
Adjustment mode of FiO2
HF3
HFlow
LFlow
60
Manual
9. Package Contents
After unpacking the system, make sure you have everything shown here (Different models of the product
may contain different components):
No.
Articles
Qty.
Notes
1
Main Device
1
2
Air Filter
2
3
Power Cord
1
4
Adapter
1
5
Accompanying Documents
1
The product’s service life is five years if the use, maintenance, cleaning and disinfection are in strict
accordance with the user manual.
Note
Airway devices are recommended for use with the High-flow nasal cannula Respiratory humidifier (HF3)
equipment and shall conform with ISO 18190:2016 and ISO 18562-1:2020.

HF3 Operator’s Manual V1.1
page 15 of 52
WARNING
This device should only be used with the accessories manufactured or recommended by Northern or
with those recommended by your prescribing physician or with the medical device registration
certificate shall be used in the device. The use of inappropriate accessories may affect the performance
of the device and impair the effectiveness of therapy.
Do not pile up the long tube at the head of the bed, as it may wrap around the head or neck of the
patient during sleep.
Do not connect any equipment to the device unless recommended by Northern or your health care
provider.
The device cannot be used in an environment with the temperature, humidity and atmospheric
pressure exceeding those specified in environmental conditions. Otherwise, it may affect the treatment
effect or damage the patient.
When accessories or other elements or components are added to the respiratory ventilation system,
the expiration pressure at the connector of the patient will rise.
Use only accessories specified in this chapter. Using other accessories may cause incorrect measured
values or equipment damage.
Disposable accessories cannot be reused. Reuse may degrade performance or cause
cross-contamination.
Check the accessories and their packages for damage. Do not use them if any sign of damage is
detected.
Parts which are intended to contact patients must comply with the biocompatibility requirement of
ISO10993-1 to prevent any adverse reactions arising from such contact.
Disposal of the accessories shall comply with the applicable waste control regulations.

HF3 Operator’s Manual V1.1
page 16 of 52
10.System Features
Fig. 10-1
Name
Function
Display
Display menus for operation, messages, monitoring data, etc.
Power on/of
Device on or off
Alarm pause
Press this button to mute the alert. However, if the problem causing the alert is not
solved, the alert will sound again two minutes later.
Navigation shuttle
button
Start treatment and adjust device settings.
Adapter
Deliver the gas; connects to the tube.
Water chamber
Observe the water level in the water chamber.

HF3 Operator’s Manual V1.1
page 17 of 52
Fig. 10-2
Name
Function
USB port
Insert the USB into this port
AC Power
An inlet for the AC power supply.
Low pressure
An inlet for the low O2source gas port
High pressure
An inlet for the high O2source gas port
Oxygen Inlet port
An inlet for the oxygen.
Air inlet
An inlet for the air
Fig. 10-3
Name
Function
Blood Oxygen
Used to Blood Oxygen connector (Function not implemented).
Proximal
Used to proximal connector.
Battery
An inlet for the DC power supply.

HF3 Operator’s Manual V1.1
page 18 of 52
Fig. 10-4
11.Preparation
11.1 Placement
Place the device on a stable flat surface or on a stand where the operators can easily access the device and
where the information displayed on the device is clearly visible. Keep the device at least 5cm away from
walls and ensure that the air inlet to the device is not blocked by bedding, curtains or other objects. The air
around the appliance must flow smoothly and be away from any heating or cooling equipment (e.g. forced
air vents, radiators, air conditioning) to ensure that the system works properly.
11.2 Installation and Connection
Please follow the steps:
1) Install a breathing filter on the air inlet of the device.
Name
Function
Sterile water
Sterile water
High flow
Equipment host
Mobile dollies
Used to Install other accessories

HF3 Operator’s Manual V1.1
page 19 of 52
2) Install the automatic humidifying water box: remove the knob caps from the two air outlets of the
humidifying water box and install the supplied adapter on the two air outlets of the humidifying water
box: install the humidifying water box on the apparatus, lightly press the apparatus heating plate, then
align the notches and slide the humidifying water box in, taking care to align the air outlets of the
oxygen therapy apparatus. Push in the humidified water cartridge firmly until the cartridge snaps into
position.
3) Connect the water bag: hang the sterile water bag on a hook so that it is 20cm above the oxygen
therapy device and insert the conical tip of the water supply pipe into the bottom of the water bag. Open
the ventilation cover next to the conical tip, water will automatically rush into the humidified water box to
the required water level and maintain the water level until the water in the bag is completely used up.
4) Connect the threaded heating line to the air outlet of the humidified water box adapter, and make the
threaded heating line snap to lock the adapter tightly.
Warnings and Precautions
Do not open the oxygenator until the humidified water cartridge is installed. Please use the
recommended humidified water cartridge with a capacity of 210 mL or more.
During use, the water in the humidifying water container can be very hot. Be cautious when removing
and emptying the humidifying water container.
Do not touch the heated base plate, the humidifying water tank or the base of the humidifying water
tank during use.
If the oxygenator is fitted with a humidified water container, avoid tilting the oxygenator to prevent water
from entering the body when moving it.
Before handling the Oxygenator, empty the water from the humidified water cartridge and ensure that
the cartridge is free of water before handling.
To ensure continuous wetting, ensure that the Oxygenator does not operate with the humidified water
cartridge as well as the water bag without water.
Check the flow of water into the humidifying water box to ensure it is below the water level, if the water
level is above the water level replace the humidifying water box immediately.
Do not alter the breathing line or nasal oxygen tube in any way.
Do not allow the breathing tube to come into direct contact with the skin for long periods of time.
Heating any breathing line or nasal oxygen tube component above room temperature (e.g. wrapping it
in a blanket, heating it in an incubator or heating it with a top heater for newborns) can lead to serious
injury.
Do not use any insulation sleeves or any similar accessories not recommended by the manufacturer
(Northern Meditec).
Please place the heated breathing line away from any electronic monitoring leads (EEG, ECG/EKG,
EMG, etc.) to reduce the possibility of any monitoring signal interference.
On the quality and purity of the water to be used in the HUMIDIFIER, and that adding other substances
can have adverse effects.
11.3 Oxygen Connection
The high flow oxygen therapy device measures the oxygen concentration at the patient delivery end via
sensors: when there is a partial blockage from the humidified water box outlet end to the patient interface

HF3 Operator’s Manual V1.1
page 20 of 52
end, the oxygen concentration will be affected for a certain period of time. High flow oxygen therapy device
oxygen source requirements: oxygen source input pressure 0.28Mpa~0.6Mpa; oxygen source
concentration >95%.
WARNING
Please ensure that the oxygen connection tube is tightly connected to the high-pressure oxygen source
and the high-pressure oxygen input of this device without any loosening or leakage to avoid oxygen
leakage affecting the normal operation of the device.
The oxygen supply must comply with the local standards of medical oxygen.
To prevent disconnection of the tubing or tubing system during use, especially during ambulatory use,
only tubes in compliance with ISO 5367 or ISO 80601‐2‐74 should be used.
The use of oxygen requires that special care be taken to reduce the risk of fire. Accordingly, for safety it
is necessary that all sources of ignition be kept away from the unit and preferably out of the room in
which it is being used. Oxygen should be located in a position or in the presence of an open flame. The
unit should be located in a position where ventilation around the unit is not restricted.
A spontaneous and violent ignition may occur if oil, grease or greasy substances come in contact with
oxygen under pressure. There substances must be kept away from all oxygen equipment.
Ensure that the HF3 is switched on before connecting oxygen.
Oxygen must only be added through the special oxygen inlet port on the back of the unit. To ensure that
oxygen enters the unit correctly, the oxygen inlet port must be fitted properly to the filter holder and the
filter holder must be fitted properly to the unit. The power cord connector should also be well secured.
Do not connect more than 80L/min O2 to the oxygen inlet port on the back of the unit.
The oxygen concentration delivered to the patient can be affected by changes to the flow setting,
oxygen setting, patient interface or if the airpath is obstructed.
When finished, turn off the oxygen source. Remove the output of the oxygen source from the oxygen
inlet port on the back of the unit. The oxygen flow must be turned off when the unit is not operating, so
that oxygen does not build up inside the device.
The oxygen analyzer within the HF3 uses ultrasonic measurement technology. It does not require
in-field calibration. It is designed for use with pure oxygen-connecting any other gases or mixtures of
gases will cause it to function incorrectly.
12.Use of Device
12.1 Start-up the device
If you have connected the device correctly, switch on the power and press and hold the On/Standby button
for 3 seconds, and the device will enter the working state. You can set the mode and parameters by
adjusting the shuttle button or by touch. Before proceeding with the treatment, you should check that the
device and accessories are intact and test to make sure that everything is in order for the air supply, alarms
etc.
Table of contents