Table of Contents
1. Safety Information ................................................................................................................................................................... 5
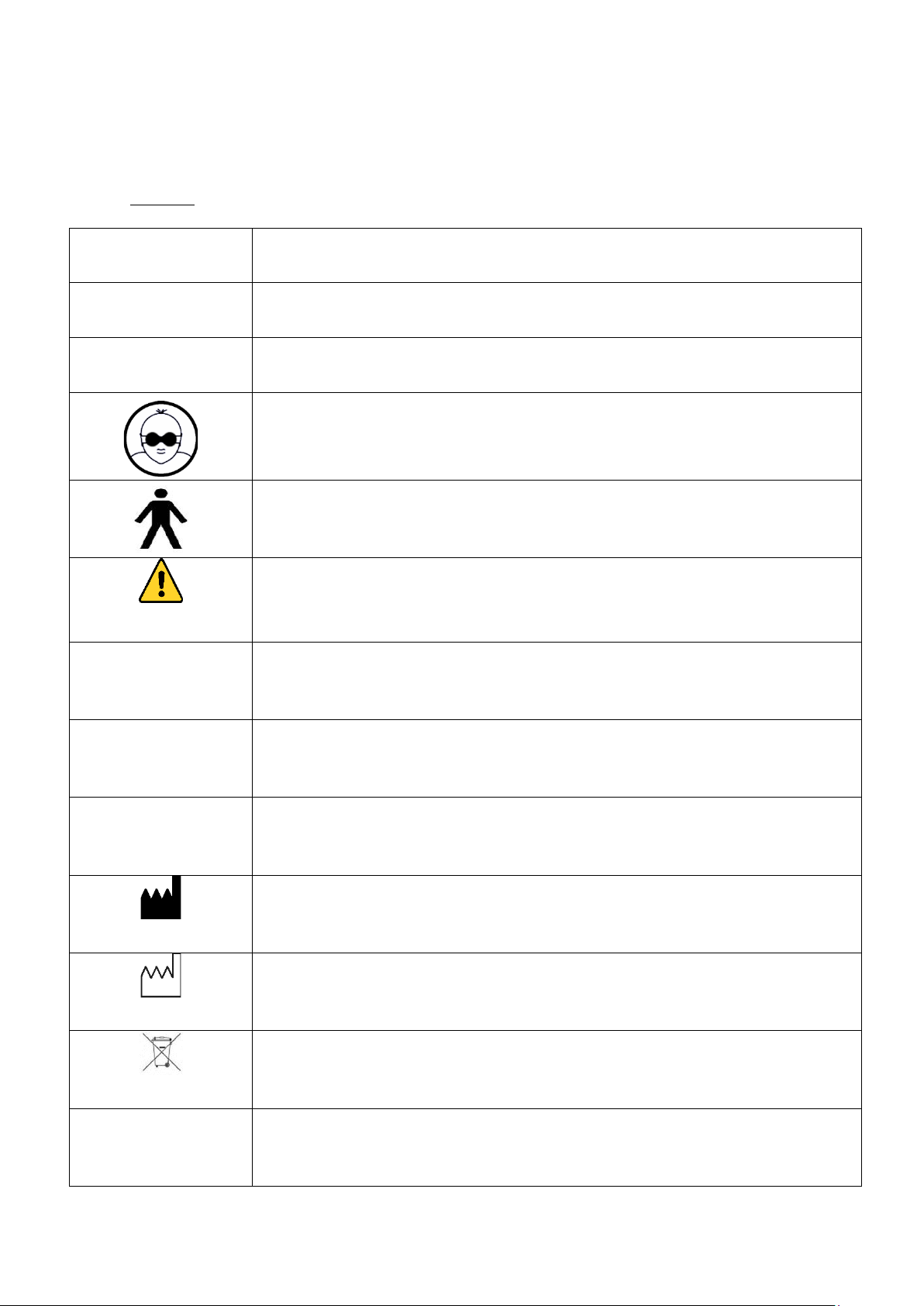
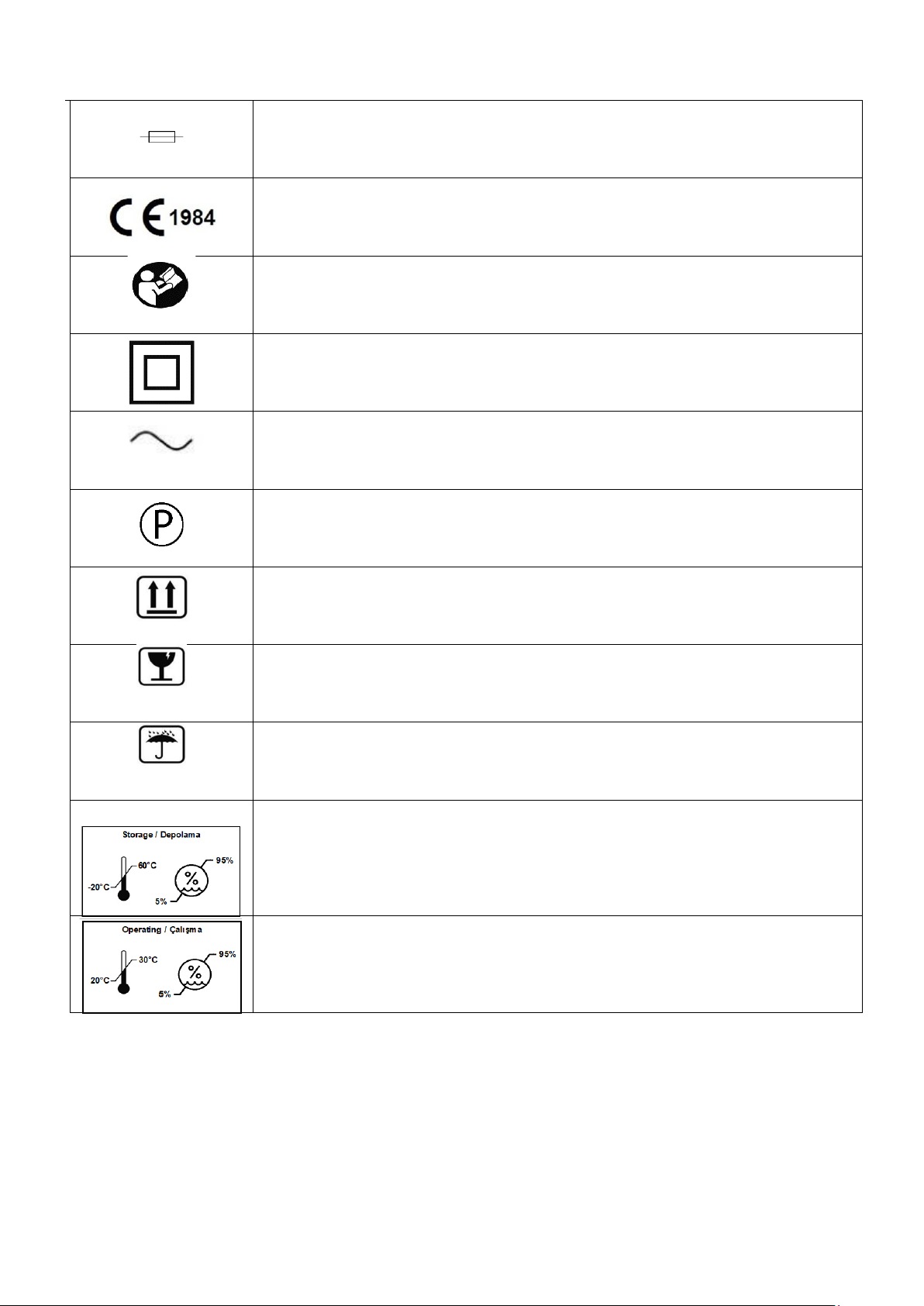
1.1. Symbols ................................................................................................................................................................................. 5
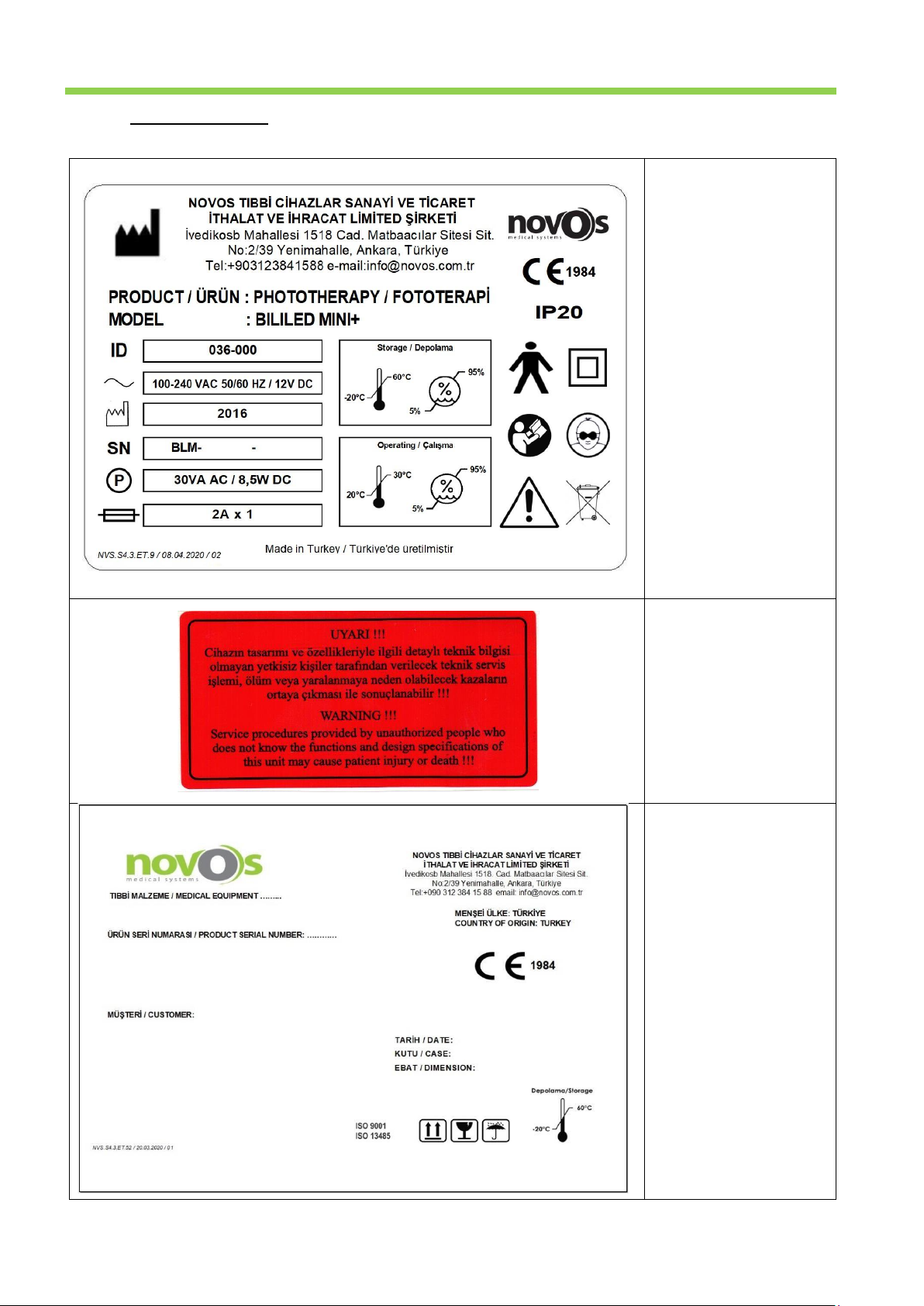
1.2. Label Information .................................................................................................................................................................. 7
1.3. User Obligations for Patient Safety ....................................................................................................................................... 8
1.4. Patient Monitoring ................................................................................................................................................................ 8
1.5. Limitation of Liabilities .......................................................................................................................................................... 8
1.6. Warranty................................................................................................................................................................................ 9
2. Product Description.................................................................................................................................................................... 10
2.1. About This Manual .............................................................................................................................................................. 10
2.1.1. Scope 10
2.1.2. Target Users ................................................................................................................................................................. 10
2.2. Applications ......................................................................................................................................................................... 10
2.2.1. Usage Purpose.............................................................................................................................................................. 10
2.2.2. Patient Population........................................................................................................................................................ 10
2.2.3. Life Cycle....................................................................................................................................................................... 10
2.3. Usage Restrictions ............................................................................................................................................................... 11
2.3.1. Operating...................................................................................................................................................................... 11
2.3.2. Power Supply................................................................................................................................................................ 11
2.3.3. Servicing ....................................................................................................................................................................... 11
2.3.4. Cleaning and Maintenance........................................................................................................................................... 11
2.3.5. Warnings Regarding Indications, Contraindications, Possible Physiological Effects .................................................... 11
2.3.6. Warnings for the Use of the Device ............................................................................................................................. 12
2.3.8. Restrictions of the Environment in which the Device will be Used.............................................................................. 13
2.3.9. Electrical Safety Restrictions ........................................................................................................................................ 13
2.3.10. Transportation Restrictions ............................................................................................................................. 14
3. Parts and Controls.................................................................................................................................................................. 15
3.1. Isometric View..................................................................................................................................................................... 15
3.2. Front View ........................................................................................................................................................................... 16
3.3. Bottom View........................................................................................................................................................................ 17
4. Preparation............................................................................................................................................................................. 18
4.1. Unpacking and Installation .................................................................................................................................................. 18
4.2. Electronic Operation Control............................................................................................................................................... 21
5. Use of Device.......................................................................................................................................................................... 21
5.1. Positioning Bililed Mini+ ...................................................................................................................................................... 21
5.2. Starting Bililed Mini+ ........................................................................................................................................................... 22