NVS.S9.KK07/Rev.07/26.10.2020
Table of Contents
1. Safety Information .......................................................................................................................................... 2
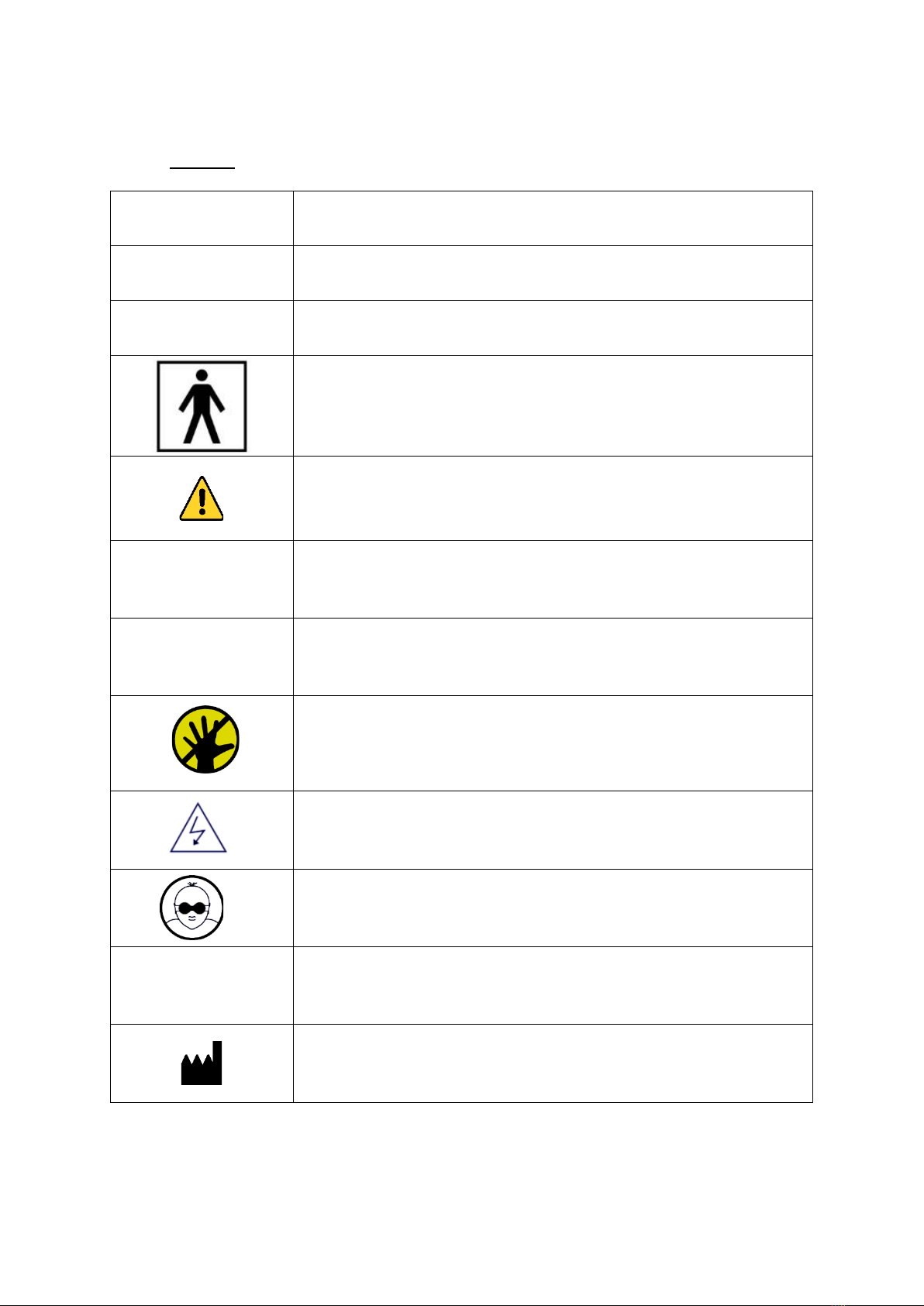
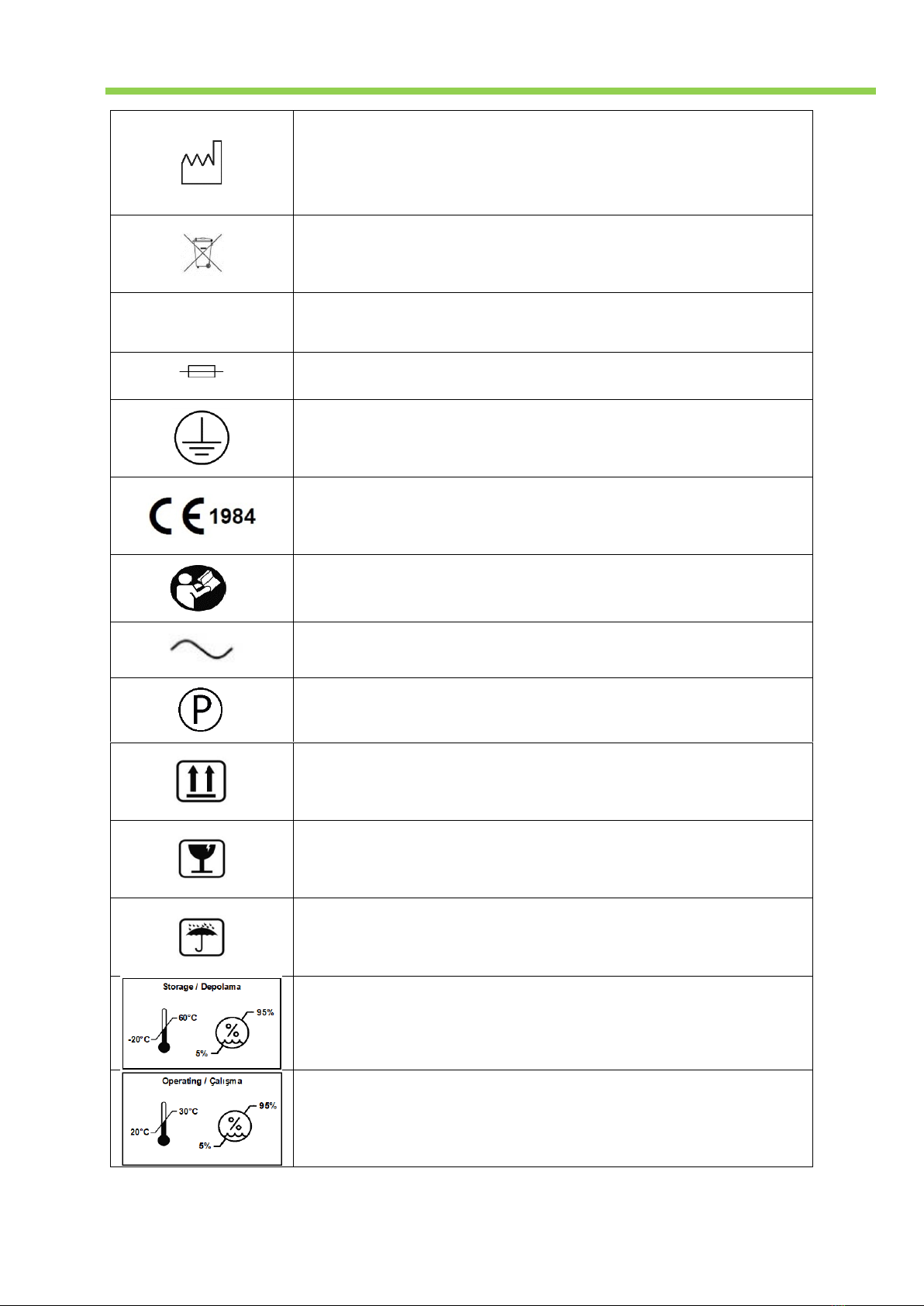
1.1. Symbols ........................................................................................................................................................ 2
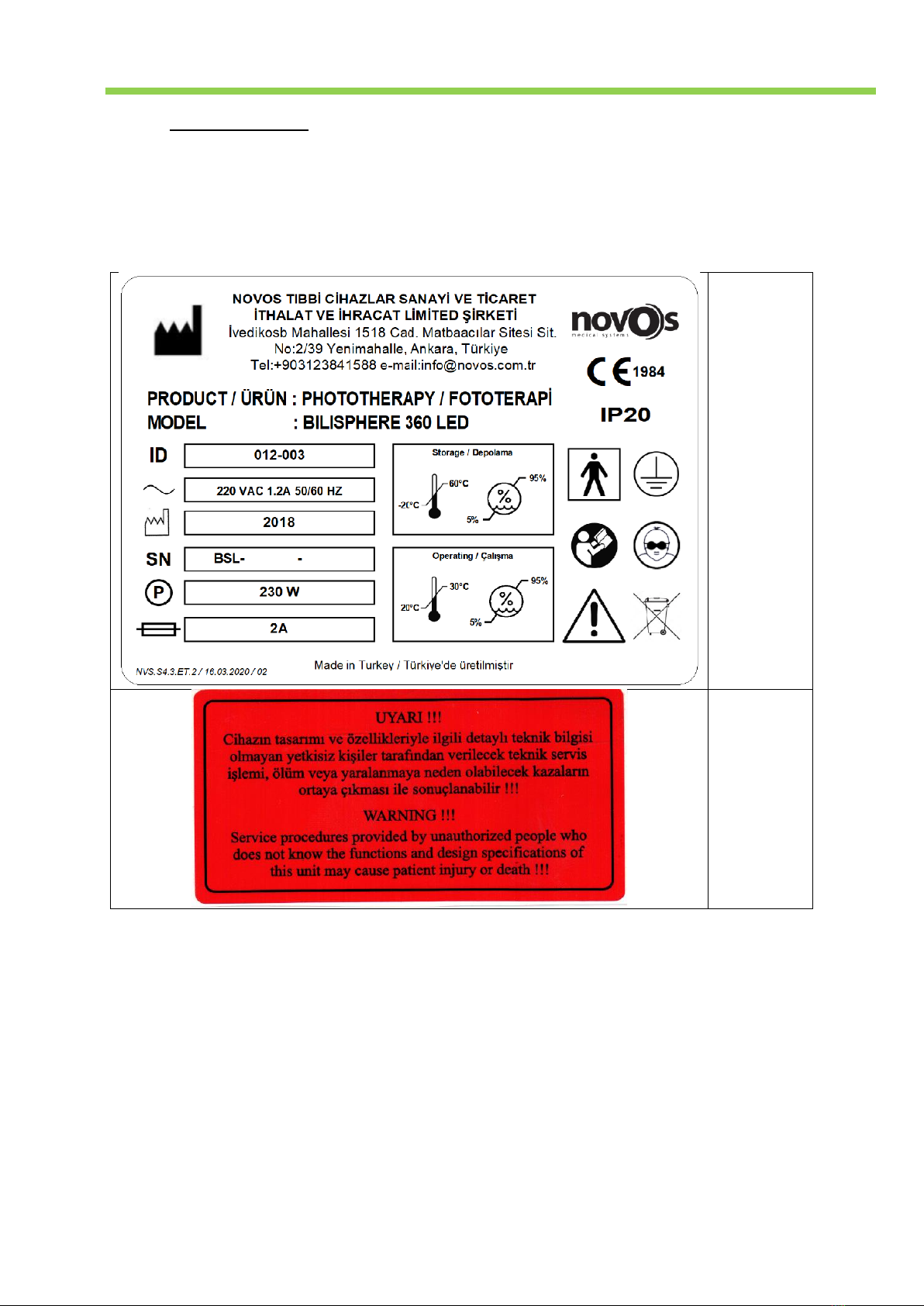
1.2. Label Information......................................................................................................................................... 4
1.3. User Obligations for Patient Safety.............................................................................................................. 6
1.4. Patient Monitoring....................................................................................................................................... 6
1.5 Limitation of Liabilities................................................................................................................................. 6
1.6 Warranty...................................................................................................................................................... 7
2. Usage Purpose................................................................................................................................................. 8
2.1. Applications.................................................................................................................................................. 8
2.2. Target Users ................................................................................................................................................. 8
2.3. Patient Population........................................................................................................................................ 8
2.4. Usage limitations.......................................................................................................................................... 9
2.4.1. Warnings Regarding Indications, Contraindications, Possible Physiological Effects........................... 10
3. Parts and Controls......................................................................................................................................... 11
3.1. Isometric View............................................................................................................................................ 11
3.2. Front View.................................................................................................................................................. 12
3.3. Side View.................................................................................................................................................... 13
3.4. Control Panel.............................................................................................................................................. 14
3.4.1. Main Display........................................................................................................................................ 14
3.5. Temperature Sensors................................................................................................................................. 14
4. Preparation ................................................................................................................................................... 15
4.1. Unpacking and Installation......................................................................................................................... 15
4.2. Electronic Operation Control ..................................................................................................................... 18
5. Use of Device................................................................................................................................................. 26
5.1. Turning On Bilisphere 360 LED................................................................................................................... 26
5.2. Use of Control Panel................................................................................................................................... 28
5.2.1. Main Display........................................................................................................................................ 28
5.2.2. Alarms and Warnings .......................................................................................................................... 29
5.2.2.1. Alerts ...................................................................................................................................... 31
5.2.2.2. Audible Alarms Activation / Deactivation............................................................................... 32
5.2.3. Settings Menu ..................................................................................................................................... 33
5.2.3.1. Changing Display Language .................................................................................................... 33
5.2.3.2. Total Lamp Usage Time .......................................................................................................... 34
5.2.3.3. Changing the Temperature Unit............................................................................................. 34
5.2.3.4. Touch Screen Calibration........................................................................................................ 35
5.2.3.5. DEMO Mode........................................................................................................................... 37
5.3. Starting Therapy......................................................................................................................................... 39