10
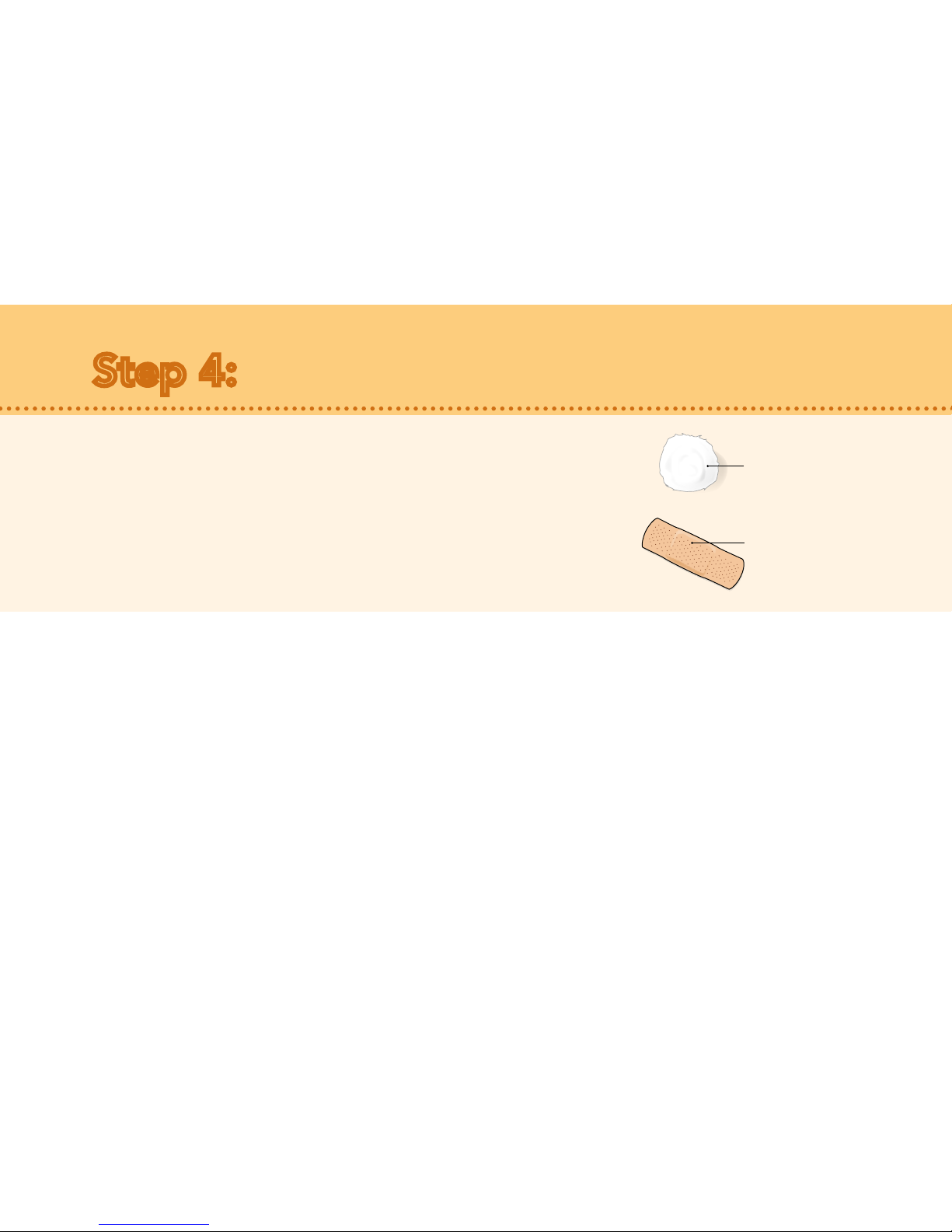
Adhesive bandage
Cotton ball or gauze
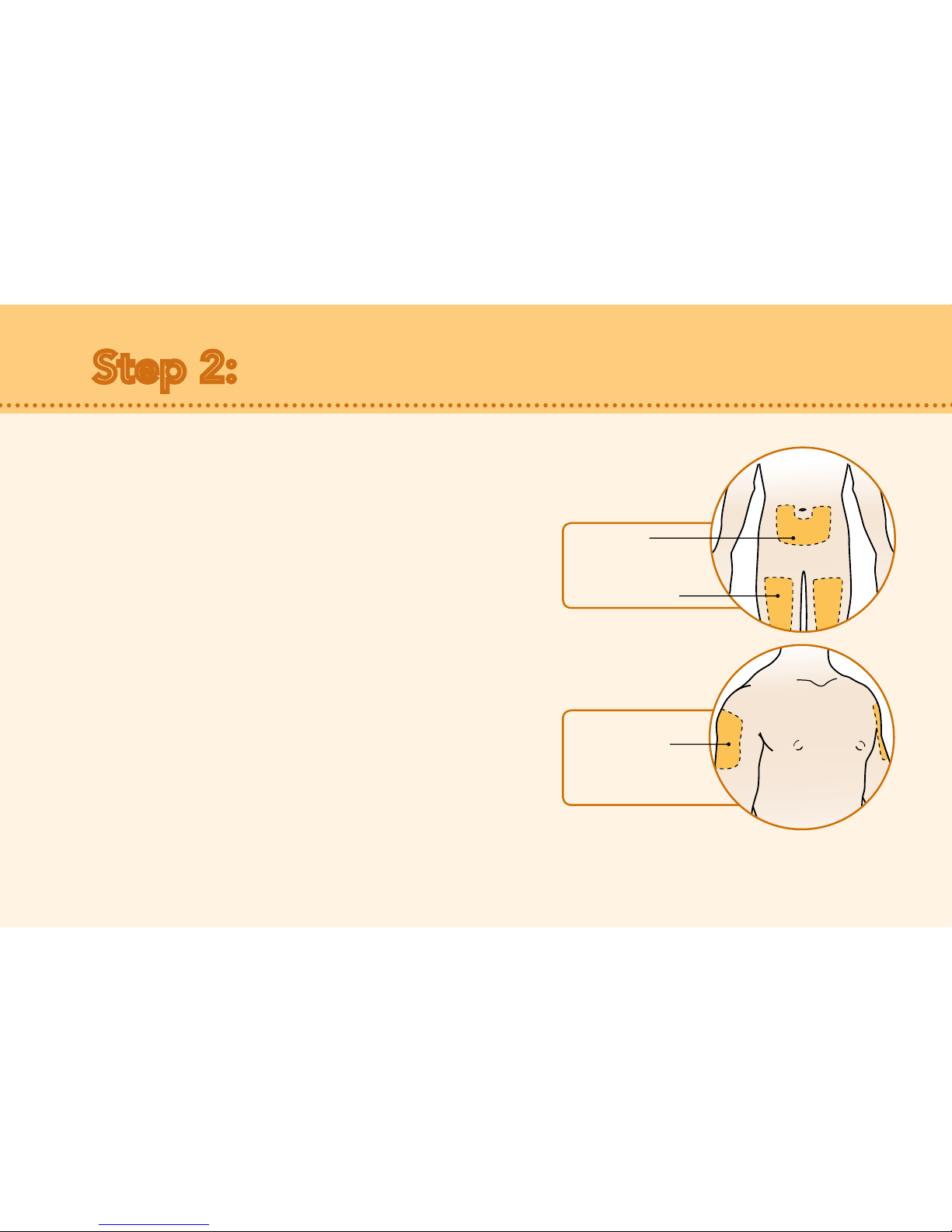
Care of injection site:
•There may be a little bleeding at the injection site. You
can press a cotton ball or gauze over the injection site.
• Do not rub the injection site.
• If needed, you may cover the injection site with an
adhesive bandage.
Step 4: After the Injection
Disposing of used ClickJect Autoinjectors:
• Put your used ClickJect Autoinjector in an FDA-cleared Sharps disposal container right away after use.
Do not throw away (dispose of) loose needles and prefilled syringes in your household trash.
• If you do not have an FDA-cleared Sharps disposal container, you may use a household container that is:
º made of a heavy-duty plastic,
º can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
º upright and stable during use,
º leak resistant, and
ºproperly labeled to warn of hazardous waste inside the container.