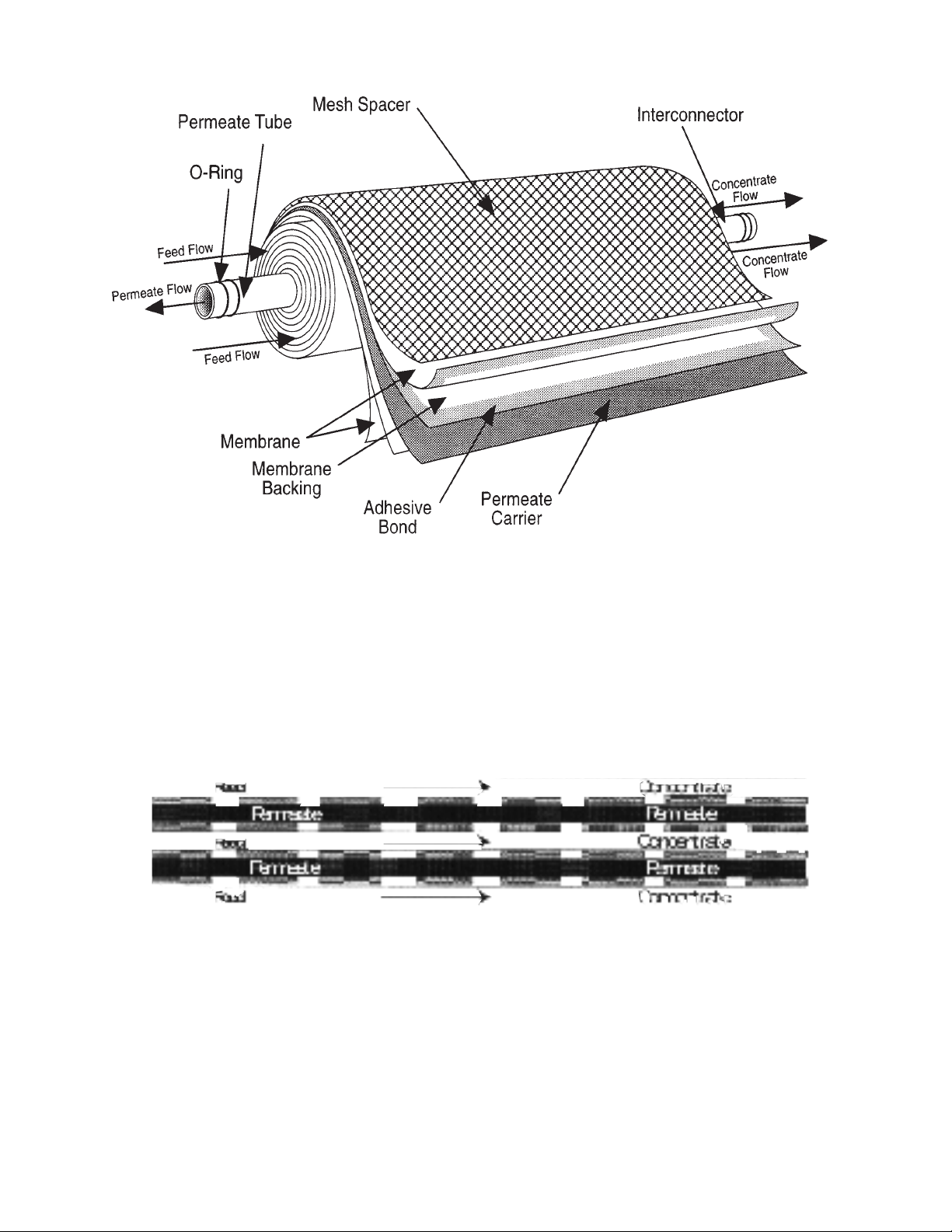
8
SALTS
CATIONS
Percent Maximum
Percent Passage Concentration
Name Symbol Rejection (Avg) Percent
Sodium Na+94-96 5 5-10
Calcium Ca+2 96-98 3 *
Magnesium Mg+2 96-98 3 *
Potassium K+1 94-96 5 5-10
Iron Fe+2 98-99 2 *
Manganese Mn+2 98-99 2 *
Aluminum Al+3 99+ 1 10-20
Ammonium NH4+1 88-95 8 3-8
Copper Cu+2 98-99 1 10-20
Nickel Ni+2 98-99 1 10-20
Zinc Zn+2 98-99 1 10-20
Strontium Sr+2 96-99 3 -
Hardness Ca & Mg 96-98 3 *
Cadmium Cd+2 96-98 3 10-20
Silver Ag+1 94-96 5 *
Mercury Hg+2 96-98 3 -
ANIONS
Chloride Cl-1 94-95 4 5-8
Bicarbonate HCO3-1 95-96 4 5-10
Sulfate SO4-2 99+ 1 5-15
Nitrate NO3-1 85-95 10 3-6
Fluoride F-1 94-96 5 5-8
Silicate SiO2-2 80-95 10 -
Phosphate PO4-3 99+ 1 10-20
Bromide Br-1 94-96 5 5-8
Borate B4O7-2 35-70** - -
Chromate CrO4-2 90-98 6 8-12
Cyanide CN-1 90-95** - 4-12
Sulfite SO3-2 98-99 1 5-15
Thiosulfate S2O3-2 99+ 1 10-20
Ferrocyanide Fe(CN)6-3 99+ 1 10-20
ORGANICS
Maximum
Molecular Percent Concentration
Weight Rejection Percent
Sucrose Sugar 34299.9 30-35
Lactose Sugar 360 99.9 30-35
Protein 10,000 Up 99.9 50-80
Glucose 180 99.0 15-20
Phenol 94 *** -
Acetic Acid 60 *** -
Formaldehyde 30 *** -
Dyes 400 to 900 99.9 -
Biochemical
Oxygen Demand (BOD) 90.0-99.9
Chemical -
Oxygen Demand (COD) 99.9
Urea 60 40-60 Reacts similar to
a salt
Bacteria & Virus 50,000 to 99.9+
500,000 -
Pyrogen 1,000 99.9+ -
to 5,000
*** Permeate is enriched in material due to preferential passage
through the membrane.
GASES, DISSOLVED
Carbon Dioxide CO230-50%
Oxygen O2Enriched in permeate
Chlorine Cl230-70%
To estimate passage of salts for membranes other than SEPA-HR, take the
passage for the SEPA-HR and multiply by the factor for the passage for the
particular membrane. The factors are:
SEPA-SR is 1.6 times SEPA-HR passage
SEPA-PR is 2.5 times SEPA-HR passage
Operation of the SEPA-HR membrane at pressures over 400 psig (27.6 bar)
will reduce salt passage slightly. Operation at 200 psig (13.8 bar) will increase
the passage of monovalent ions by approximately 2.0 times and the passage of
multivalent ions will increase by 1.5 times the 400 psig (27.6 bar) passage.
For SEPA membranes with larger pores than the SEPA-PR it is recommended
that actual tests be run prior to estimating the permeate quality.
The maximum concentrations given in the table are the approximate
concentrations resulting in an osmotic pressure of 500 psi (34.5 bar) for the
solution.
Compounds such as CaSO4which have specific solubility limits can be
controlled with proper addition of dispersants. Check with the factory for
more information on Osmonics’ special line of dispersants.
* Must watch for precipitation; other ion controls maximum
concentration
** Extremely dependent on pH; tends to be an exception to the
rule
The following are typical rejections and passages for various salts and organics
using the SEPA®-HR membrane at 400 psig (27.6 bar) operating pressure.
Modules made with this membrane, such as the OSMO®-HR, can be expected
to give these same passages. As can be seen, multivalent ions tend to have
less passage than do monovalent ions. If monovalent ions are combined with
multivalent ions to form a salt, the passage will be controlled by the multiva-
lent ion. In RO all ions must be combined as the salt form before passages can
be considered.
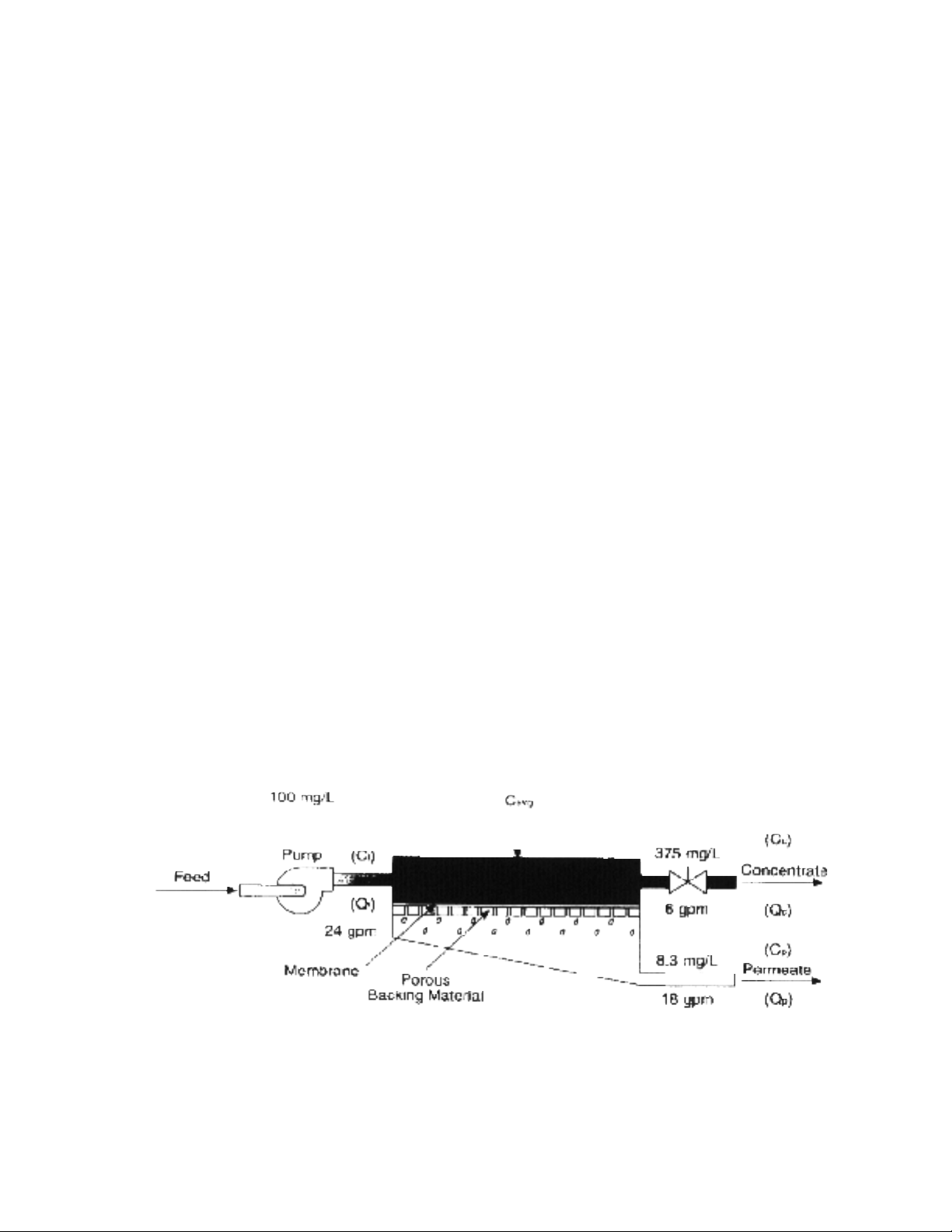
For estimating purposes, to obtain the expected permeate quality when han-
dling a solution of salts, take a simple average of the feed concentration and
the
concentrate concentration and multiply this figure by the average percent pas-
sage to calculate the average concentration of the permeate.Salts or organics
that are complexed with organics of large molecular weights will tend to act
like the organics with which they are complexed.
NOTE: The actual permeate water quality will vary with the inlet water quality and can only be veri-
fied by actual analysis of the permeate stream.
Figure 5 - Typical Membrane Rejections/Passages