OT Bioelettronica NOD User manual

User Manual v1.3
NOD
Three functions, one device
Read this manual carefully before using
NOD

2

3
1 GENERAL DESCRIPTION…………………………….5
2 NOD KIT CONTENT……………………………………4
3 END USER……………………………………………….5
3.1 CONTRAINDICATIONS………………………………………………5
3.2 SIDE EFFECTS……………………………………………..………..5
4 SAFETY PRECAUTIONS AND WARNING………….6
5 SYMBOLS USED ON NOD AND IN USER MANUAL7
6 TECHNICAL SPECIFICATIONS……………………..8
7 DETAILED DESCRIPTION …………………………10
7.1 CONTROLS,INDICATORS AND ADAPTERS……………………..10
7.1.1 LEDS INDICATORS…………………………………………….10
7.1.2 ON/OFF SWITCH…………………………………………….11
7.1.3 CONNECTORS AND ADAPTERS………………………………11
7.2 SYSTEM REQUIREMENTS FOR SMARTPHONES/TABLETS…….12
7.3 CHARGING THE NOD…………………………………………… 12
7.4 USE OF NOD………………………………………..…………….12
8 USE OF NOD .......................................................13
8.1 BIOFEEDBACK (ISOMETRIC MUSCLE TESTING)…………..…13
8.2 DYNAMOMETER (CRANIO CERVICAL FLEXION TEST) ........ 13
8.3 ALGOMETER (PAIN TEST) ......................................... 14
8.4METHODS OF USE ................................................... 15
8.4.1 DOWNLOAD THE NOD APP .................................... 15
8.4.2 PAIRING SMARTPHONE /TABLET AND NOD DEVICE ...... 16
8.4.3 FIRST USE….……………………………………………………18
8.4.4 USAGE FUNCTIONS............................................... 19
9 TROUBLESHOOTING..........................................30
10 NOD MAINTENANCE AND STORAGE...............30
11 RISK ANALYSIS..............................................29
12 TECHNICAL CHARACTERISTICS .....................30
13 WARRANTY ......................................................35

4
1GENERAL DESCRIPTION
The NOD device is a generic 1-channel amplifier.
This system can amplify and filter any differential
and single ended signal.
NOD is a multi-purpose tool that combines the
functionality of three different tools:
1) Biofeedback, provides real-time feedback on
the force exerted by the patient during an exercise
to allow you to correctly perform rehabilitation
protocols.
2) Dynamometer, can measure the traction and
compression force produced by the patient during
an exercise and allow comparisons in different
measurement sessions.
3) Algometer, allows to mimic the acupressure
action used to measure pain in a specific body
district.
The information collected from the input is
conditioned and transferred to a smartphone /
tablet via wireless communication (Bluetooth®).
In Bluetooth® wireless mode, the NOD device can
be used with an App, called Physio (available on
Google Play Store) to view the detected data.
2NOD KIT CONTENT
•1 portable mono-channel amplifier NOD;
•1 USB-C cable;
•1 Algometer tip;
•1 Pad;
•1 Magnetic pad.

5
3END USER
NOD
is intended to be used in an outpatient physi-
otherapeutic or medical environment, so that the
medical staff can use the information detected by
the device to perform a diagnosis.
Type of users are specialized operators:
a) Background: Minimum. Basic physics
b) Language Understanding: Basic English
c) Experience: Minimum. Minimum training for
the use of the device
d) Eligible impairments:
•40% maximum hearing reduction with 60%
residual hearing;
•40% sight reduction with 60% residual sight
3.1 CONTRAINDICATIONS
NOD
has no contraindications when used jointly
with a smartphone or tablet, provided that all the
electrical devices connected to it and the power line
comply with safety rules and standards concerning
grounding and leakage currents.
3.2 SIDE EFFECTS
The materials used for manufacturing all the parts
in contact with patient are biocompatible. Possible
slight cutaneous allergic reactions (e.g. skin red-
dening) are reduced to a minimum during short du-
ration of signal acquisitions. No significant side ef-
fects are known.

6
4SAFETY PRECAUTIONS AND
WARNINGS
The use of the NOD 1-channel amplifier is
absolutely forbidden in the following conditions:
•Simultaneous or near use of electrosurgery
systems, a shortwave or microwave therapy
device.
•From people unable to understand and / or
will.
•When the system is visibly damaged.
•In the presence of flammable anaesthetics
mixtures with air, oxygen or nitrous oxide.
The following precautions should be observed:
•Contact the manufacturer immediately if any
material enters the device (liquids, etc.). In
case of strong shocks impact (falling on the
floor, etc.), check the integrity of the device
after the impact. If in doubt, please contact the
manufacturer.
•The NOD device may be sensitive to electro-
magnetic interference from other devices that
could alter the force measurements and, con-
sequently, the variables calculated based on
the data collected. Therefore, we recommend
that you do not use the NOD device near de-
vices that could cause the problems described
above such as mobile phones, instrumentation
with large transformers, etc. ...).
•The operator must ensure that the battery of
the appliance has been fully recharged, as in-
dicated in this user manual, before using the
device.
•DO NOT leave the device within reach of chil-
dren or incapable people without supervision.

7
•DO NOT clean the device using acetone, ether,
freon, petroleum derivatives or other solvents.
•DO NOT use soap or water on the connector
pins.
•DO NOT clean the NOD device or connect ca-
bles by immersion, autoclave or steam clean-
ing.
5SYMBOLS USED ON NOD AND IN THE
USER MANUAL
Serial number
Manufacturer
Attention read the attached documents
before starting the device
CE marking - Device compliant with
applicable Community directives
Read the instructions
USB Indicates the USB input
ON/OFF Power button
STATUS Indicates the device’s status
Do not dispose of this product as non-
differentiated waste. Prepare the re-use
or separate collection of the product
according to the provisions of Directive
2002/96 / EC of the European Parliament
and of the Council of the European
Union on the disposal of electrical and
electronic equipment

8
The
NOD
device has been tested with reference to
the EN 60601-1 and EN 60601-1-2 Standards. If
the user connects to the
NOD
another instrumen-
tation not previously validated for joint use accord-
ing to the EN 60601-1 and EN 60601-1-2 Regula-
tions, he must ensure that the coupling between
the two devices meets the requirements of the
Regulations mentioned above.
Otherwise OT Bioelettronica declines any responsi-
bility. Regarding the functionality of the software,
please refer to the specific manual provided.
6TECHNICAL SPECIFICATIONS
The
NOD
system is a battery-powered device
designed according to medical regulations to
guarantee patient safety.
The signals picked up and amplified by the system
are then transferred to a Smartphone / Tablet
wirelessly (Bluetooth®). Table 6.1 shows the
technical specifications of the
NOD
device.

9
Model
NOD
Classification
Battery powered system
Classification code
IP20
Housing
Painted Plexiglas case
Power Supply
3,7V rechargeable battery
Battery life
8 hours (full charge)
Charging time
2 hours
Number of channels
1
Dynamic
0 ÷ 50 mVPP
Band
0 ÷ 34Hz
Input noise
< 20 VRMS
Amplification (digital)
942 V/V
Output range
0 ÷ 5 V
Resolution of the A/D
converter
16 bit
Wireless transmission
model
Bluetooth®
Sampling rate
100 Hz
Dimensions
79 x 194 x 17 mm
Weight
300g
Tab. 6.1: Technical specifications of the NOD system

10
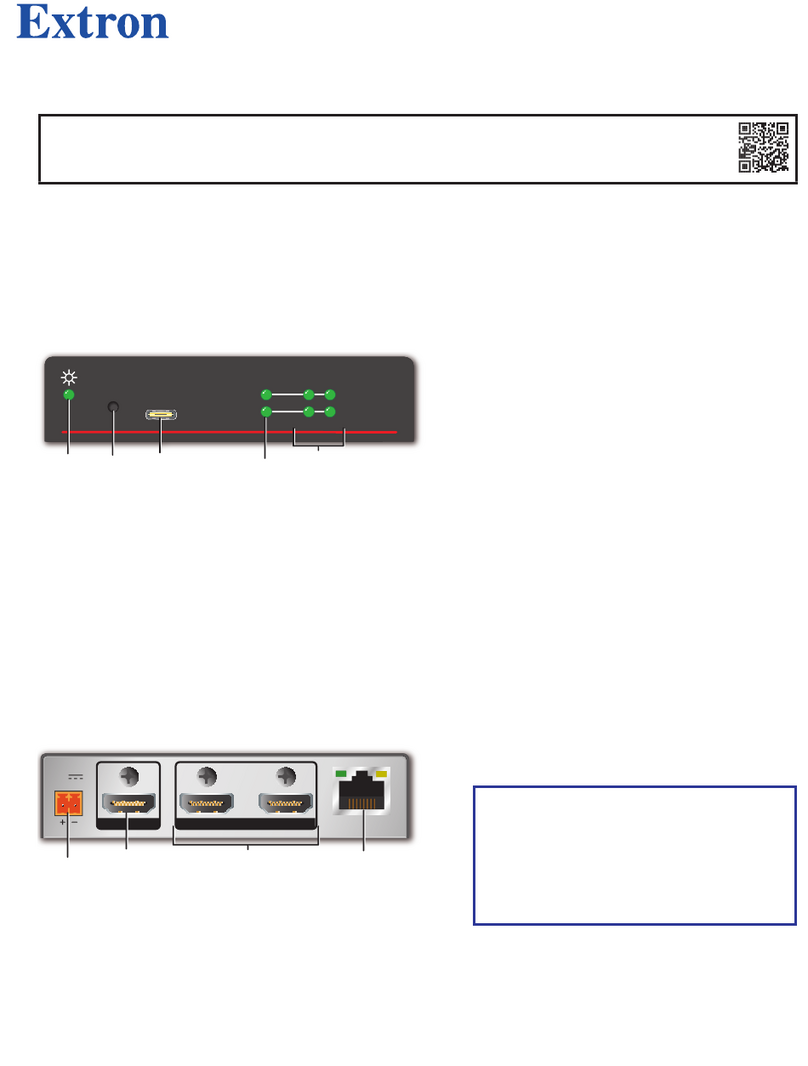
7DETAILED DESCRIPTION
Figure 7.1 shows the controls, indicators and
connectors on the NOD system and described in
the following section.
Fig. 7.1: Front panel and side view of the NOD system.
7.1 CONTROLS,INDICATORS AND ADAPTERS
Description of controls, indicators and connectors
shown in figure 7.1:
7.1.1 LED INDICATORS
Battery/Error/Status LED: This two-colour LED
(Red or Green) performs multiple functions:
•Status:
1. flashes Green when the system is
running and is waiting for a connec-
tion;
2. it remains lit Green when the sys-
tem is running and is connected to a
smartphone / tablet via the app.
Front view
Side view
USB Connector
Battery/Error/Status LED
Switch ON/OFF

11
•Battery:
1. blinks between Green and Red
when it is not connected and dis-
charged;
2. it remains lit Green with a Red LED
flashing intermittently when the
system is connected to a smartphone
/ tablet and is also discharged;
3. blinks between Green and Red
also when it is charging;
4. flashes Green when the charge is
complete.
•Errors:
1. blinks between Green and Red
when there is a communication prob-
lem via the smartphone / tablet (try
restarting the NOD);
2. flashes Red when the connection
with the smartphone or tablet has
been lost.
7.1.2 ON/OFF SWITCH
The NOD system turns on and off using the ON/OFF
button.
7.1.3 CONNECTORS AND ADAPTERS
USB connector: this connector is used to
recharge the battery.
USB cable for battery charging: The NOD
device is equipped with a USB adapter called
CUSB03 (USB C). This cable has the function to
recharge the battery of the device once connected
to a PC or a power supply with a USB output.

12
Note. If the NOD device is charged while turned
off, the LED will not light up, but the device will
anyway be charging.
7.2 SYSTEM REQUIREMENTS FOR SMARTPHONES/
TABLETS
1. Minimum Android version 4.4 (KitKat)
2. Bluetooth® from 2.1
7.3 CHARGING THE NOD
Behaviour of the device during charging
During the charging phase, the red “Recharge” LED
located on the right side of the NOD device
(posterior view) flashes red until it is recharged,
then the LED turns off.
Charge the device for at least 2 hours
before using it.
7.4 Use of NOD
Application and data acquisition with the
NOD System
To correctly apply the NOD system, proceed as
follows:
Smartphone / Tablet
•pair the NOD device to the Smartphone or
Tablet via Bluetooth®;
•launch the Physio application;
•select from the device list the name of the NOD
device (only the first time);
•select the desired functionality;
•if necessary, reset the offset via the App and
proceed with signal acquisition.

13
8USE OF NOD
NOD is intended to be used as a force detection
system generated by load cells or transducers with
differential output and single ended.
PRINCIPLE OF OPERATION
The device, called NOD, is a dynamometer capable
of detecting force when it is applied perpendicular
to its front surface. The detected force is transmit-
ted via Bluetooth® connection to a smartphone /
tablet where a dedicated application will allow the
display and recording of the force. Force display
and recording modes depend on clinical applica-
tions. The device has been developed to be
hand-held by an operator.
CLINICAL APPLICATIONS
NOD is a non-invasive clinical device for the evalu-
ation and treatment of patients with neuromuscular
problems characterized by muscular hyposthenia
and / or musculoskeletal pain. The device is in-
tended to come into contact with the patient only
temporarily and at an epidermal level on intact and
healthy skin. Three different clinical applications of
the device are possible. Patients should be properly
informed about the purposes and modalities of clin-
ical applications and these should be conducted un-
der the supervision of a physiotherapist or a physi-
cian.
8.1 BIOFEEDBACK (ISOMETRIC MUSCLE TESTING)
This function provides real-time feedback of the
force produced during a muscle contraction. This
function can be used to train muscles in all regions
of the body including the cranio-cervical flexor
muscles. The operator before performing the test,

14
instructs the patient to assume a standardized po-
sition that will allow the selective activation of a
muscle group. The operator will place the NOD on
the patient's body (at a specific point) and the latter
will have to progressively push against the front
surface of the NOD until reaching his/her maximum
strength. The operator must ensure that no joint
movements occur.
8.2 DYNAMOMETER (CRANIO CERVICAL FLEXION
TEST)
Cranio Cervical Flexion Test (CCFT) is a clinical ex-
amination for the evaluation of neuromotor control
of the deep flexors of the cervical area. The test is
indicated in patients with cervicalgia (subacute and
chronic), whiplash and cervicogenic headache. The
patient should be lying on a medical bed in a supine
position. The cervical district must be in a neutral
position, the chin and the forehead must be aligned
on a horizontal (imaginary) plane parallel to the
couch.
The operator positions the NOD, with the appropri-
ate support, behind the neck so that it adheres to
the occiput. The patient is instructed to increase
pressure on the NOD progressively by moving
his/her head vertically, as if nodding.
8.3 ALGOMETER (PAIN TEST)
The algometer provides a clinical and research tool
for the assessment of pain perception. Digital al-
gometry provides a reliable and objective quantifi-
cation of the pressure pain threshold.
After positioning the adapter on the front of the
NOD device, the operator will position the adapter

15
tip (1 cm2) perpendicularly on the anatomical struc-
ture to be evaluated. Then the pressure will grad-
ually increase until it causes pain. The patient
should be instructed to stop the operator as soon
as the pressure causes the appearance of the pain.
The pain threshold, defined as the minimum pres-
sure needed to evoke pain, will be measured in
Kg/cm2.
8.4 METHODS OF USE
8.4.1 DOWNLOAD THE NOD APP
•Click on the Google Play Store icon
•Write the word "nod ot bioelettronica" in the
search bar
•Click on the NOD App icon (OT Bioelettronica)
•Click on “install” icon and wait for the end of
the process
16
•Exit Google Play Store and proceed with the
next step
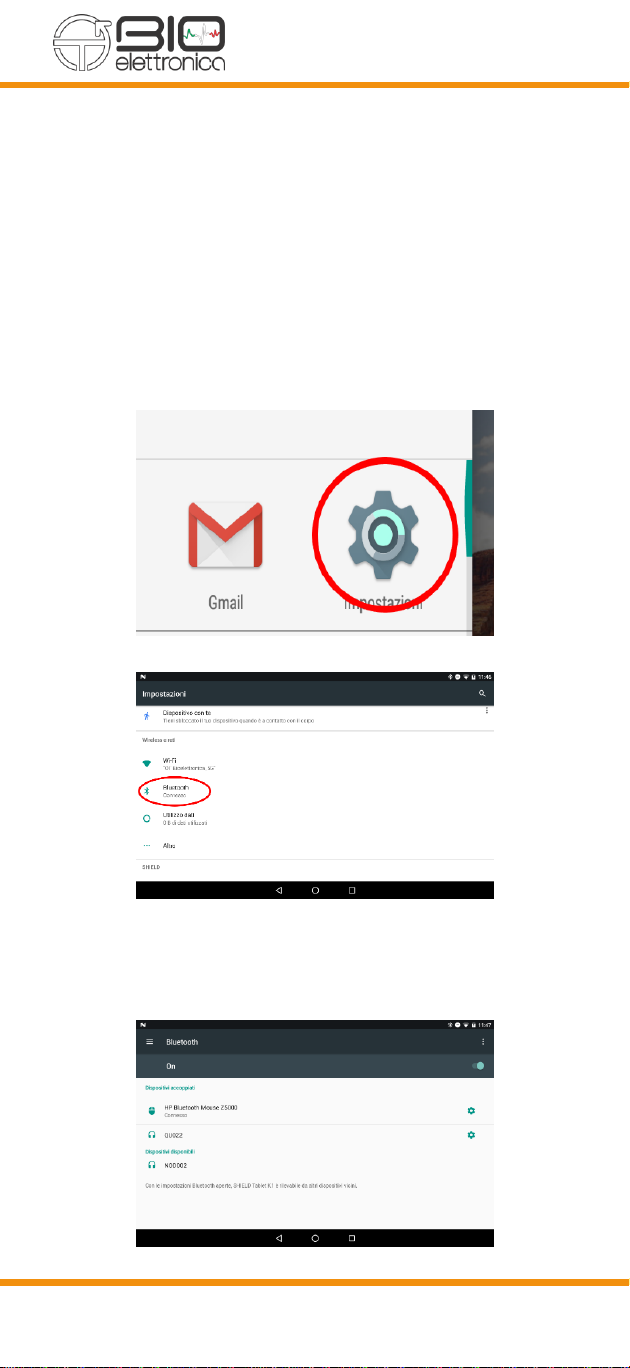
8.4.2 PAIRING SMARTPHONE /TABLET AND NOD
DEVICE
•Turn on the NOD device
•Click on the settings icon of your Android de-
vice
•Press the “Bluetooth®” button
•Search for the NODxxx device and then click
on it

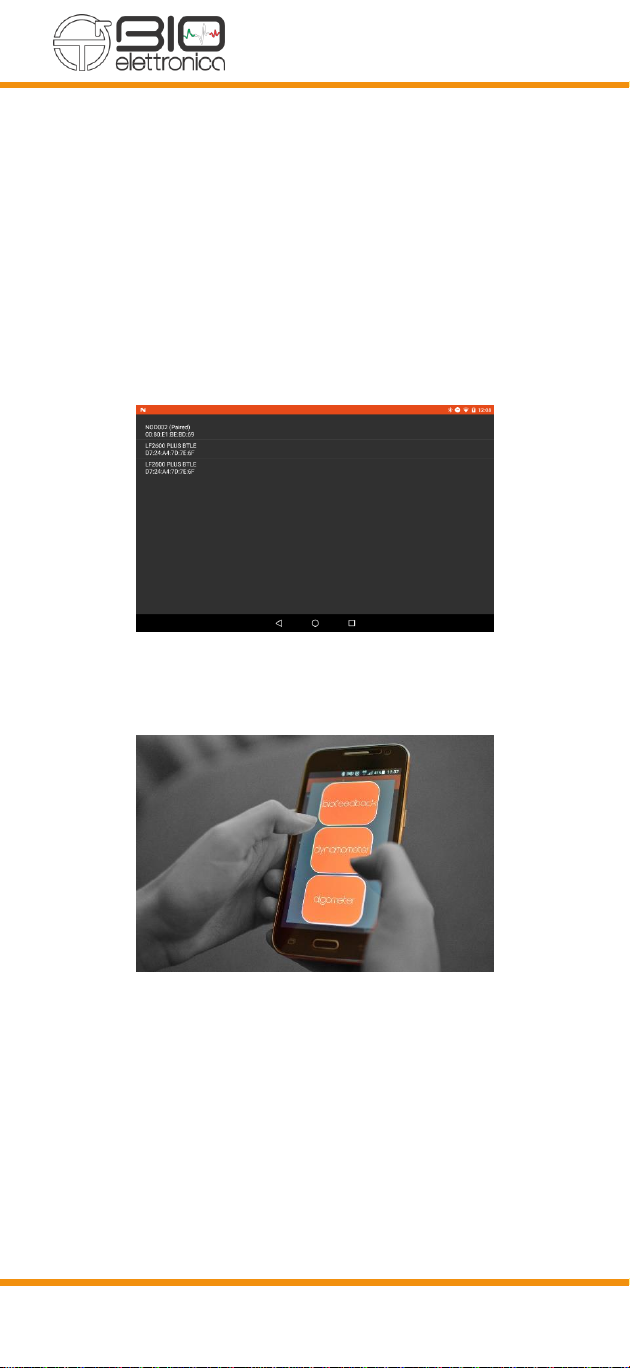
17
•Click on “pair”
•Check that the NODxxx device is present
among the paired devices and NOT on the
available devices
•Return to the main screen and proceed to the
next step.
18
8.4.3 FIRST USE
•Turn on the NOD device
•Launch the NOD App
•Allow all the required permissions
•Click on NODxxx (Paired) and wait for the con-
nection
•Select the required function

19
If it is not visible NODxxx (Paired) click on the
Android button ( ), select the required
function, open the side menu bar and then press
“Connect”. Then click on NODxxx.
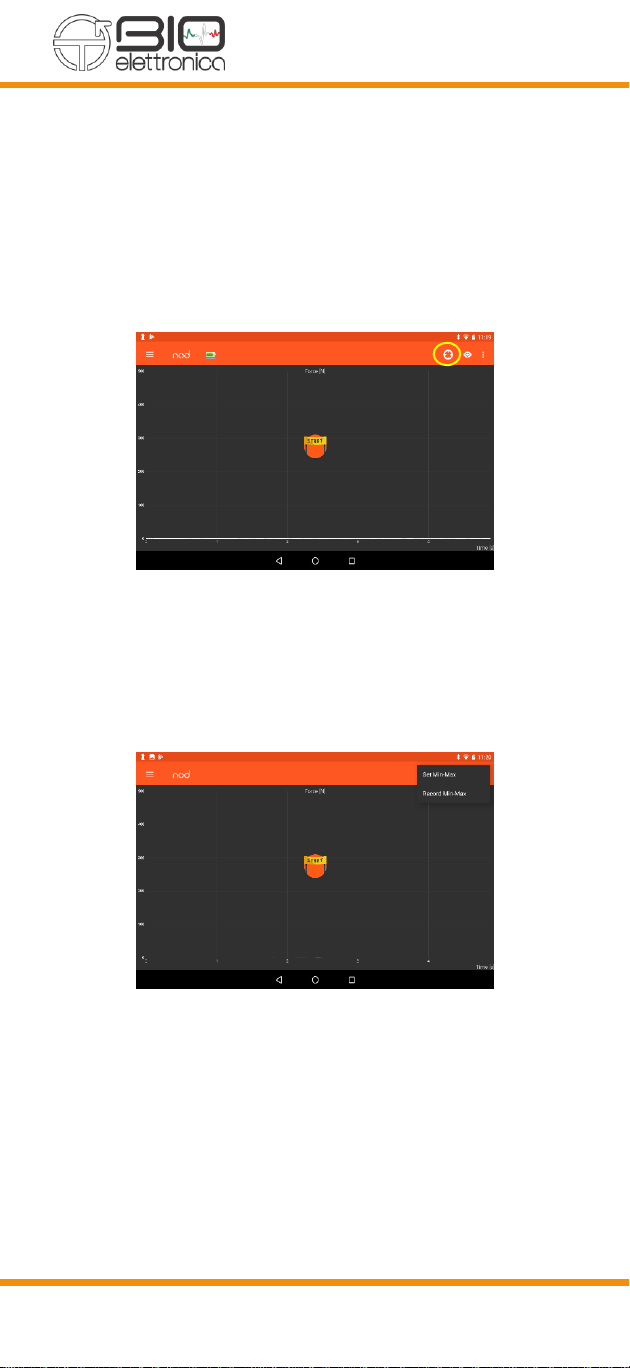
8.4.4 USAGE FUNCTIONS
In the NOD App you can use three different modes:
Dynamometer, Force Biofeedback and Algometer.
20
•Biofeedback (IST)
•Place the magnetized Magnetic pad over the
NOD device and then press the Calibration but-
ton, if there is an offset it will be reset. The
yellow circle in the figure below shows the Cal-
ibration button.
•You can set manually the Maximum Voluntary
Contraction value (MVC) o record it. To record
the MVC value click on the “Rec” button when
you are ready.
Table of contents
Other OT Bioelettronica Amplifier manuals

OT Bioelettronica
OT Bioelettronica EMG-USB2 User manual

OT Bioelettronica
OT Bioelettronica GPA4 User manual

OT Bioelettronica
OT Bioelettronica OT-BridgeAmp4 User manual

OT Bioelettronica
OT Bioelettronica Sessantaquattro+ User manual

OT Bioelettronica
OT Bioelettronica Quattro User manual

OT Bioelettronica
OT Bioelettronica FORZA User manual