4053154 r17
Product Disposal
Contact your local authorized dealer for proper disposal of the
device to ensure compliance with your local environmental
regulations.
Interference with Electromedical Devices
To guarantee the operational safety of electromedical
devices, it is recommended that the operation of mobile radio
telephones in the medical practice or hospital be prohibited.
Strong EMI sources such as electro surgery units or x-ray units
may affect performance. If performance problems occur, move
the unit to another electrical circuit or physical location.
Incompatible Units or Accessories
Incompatible Units or Accessories: To guarantee the operational
safety and function of this device, the use of unapproved units
or accessories is not advised. Doing so could result in potential
hazard. Using accessory equipment not complying with the
equivalent safety requirements of this equipment may lead to
a reduced level of safety of the resulting system. Connecting
electrical equipment to multiple socket outlets effectively leads
to creating an ME SYSTEM, and can result in a reduced level of
IEC 60601-1-1 or IEC 60601-1:2005
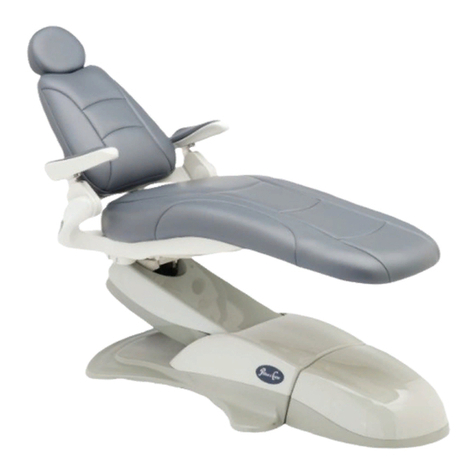
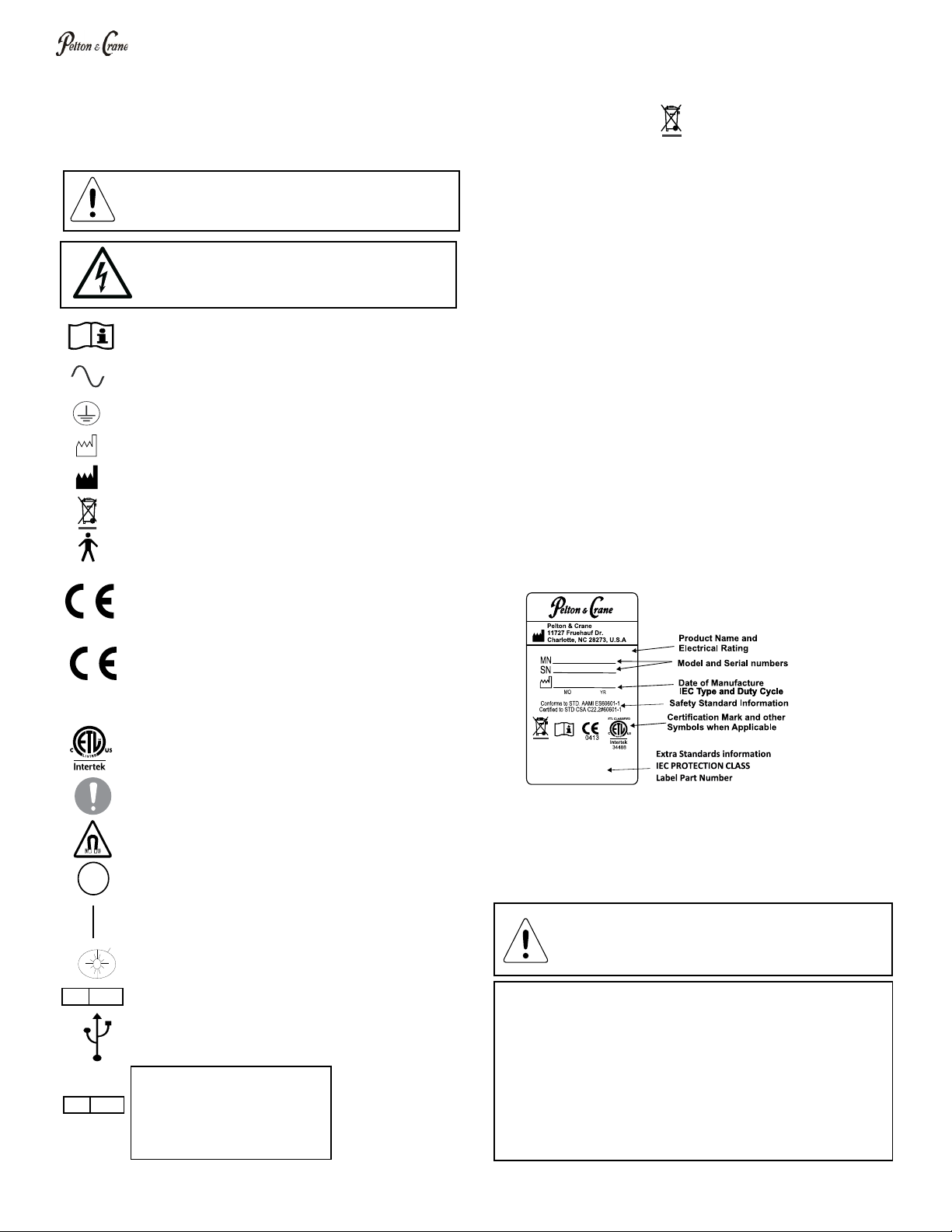
Product Identication
label states the unit model and serial number, electrical
the SAMPLE label shown below.
Working Environment
Recommended working condition is:
Ambient Temperature: 68°F to 76°F (20°C to 25°C)
Relative Humidity: 20% to 60% non-condensing
Atmospheric Pressure: 13.1 to 15.3 PSI (900 to 1060hPa)
GENERAL INFORMATION
Denition of Symbols
The following symbols and terms may be used throughout this
manual and your equipment:
WARNING: Failure to carefully follow the described
procedure may result in damage to the equipment
and/or injury to the patient/operator.
Risk of electrical shock present.
Make sure power is disconnected before
attempting this procedure.
See operating instructions.
(AC) Alternating current.
Protective earth (Ground)
Manufacturing Date
Waste Electrical and Electronic Equipment.
Type B Applied part.
Conforms with the Essential Requirements of the
European Medical Device Directive 93/42/EEC for
Class I Devices.
Conforms with the Essential Requirements of the
European Medical Device Directive 93/42/EEC for
Class IIa Devices.
Indicates conformity to General Requirements for
General mandatory action required, important to
Off
On
Light Switch
European Authorized Representative
USB Port
EC REP
Medical Device & QA Services Ltd.
76, Stockport Road,
Timperley.
Cheshire, WA15 7SN.
United Kingdom.
Tel: +44 (0) 845 527 5078 Fax: +44 (0) 161 903 9787
Email: info@ mdqas.com www.mdqas.com
EC REP
Medical Device & QA Services Ltd.
76, Stockport Road,
Timperley.
Cheshire, WA15 7SN.
United Kingdom.
Tel: +44 (0) 845 527 5078 Fax: +44 (0) 161 903 9787
Email: info@ mdqas.com www.mdqas.com
Authorized Representative:
Kaltenbach & Voigt GmbH
Bismarckring 39
88400 Biberach
Germany
WARNING: It is not safe to use the unit where there
and heavy property damages
Manufacturing Place
Storage Conditions: The device is appropriately
packaged in a box. If product is to be stored before
installation, storage and handling instructions in the
packaging should be adhered to. Handling and storage
conditions are marked on the box.
Temperature: -4°F to 122°F/ -20°C to 50°C
Relative Humidity: 10% to 90%
If the device is not to be used for some time, ensure the
master switch is switched off.
DENTAL ___ __VAC__A__HZ