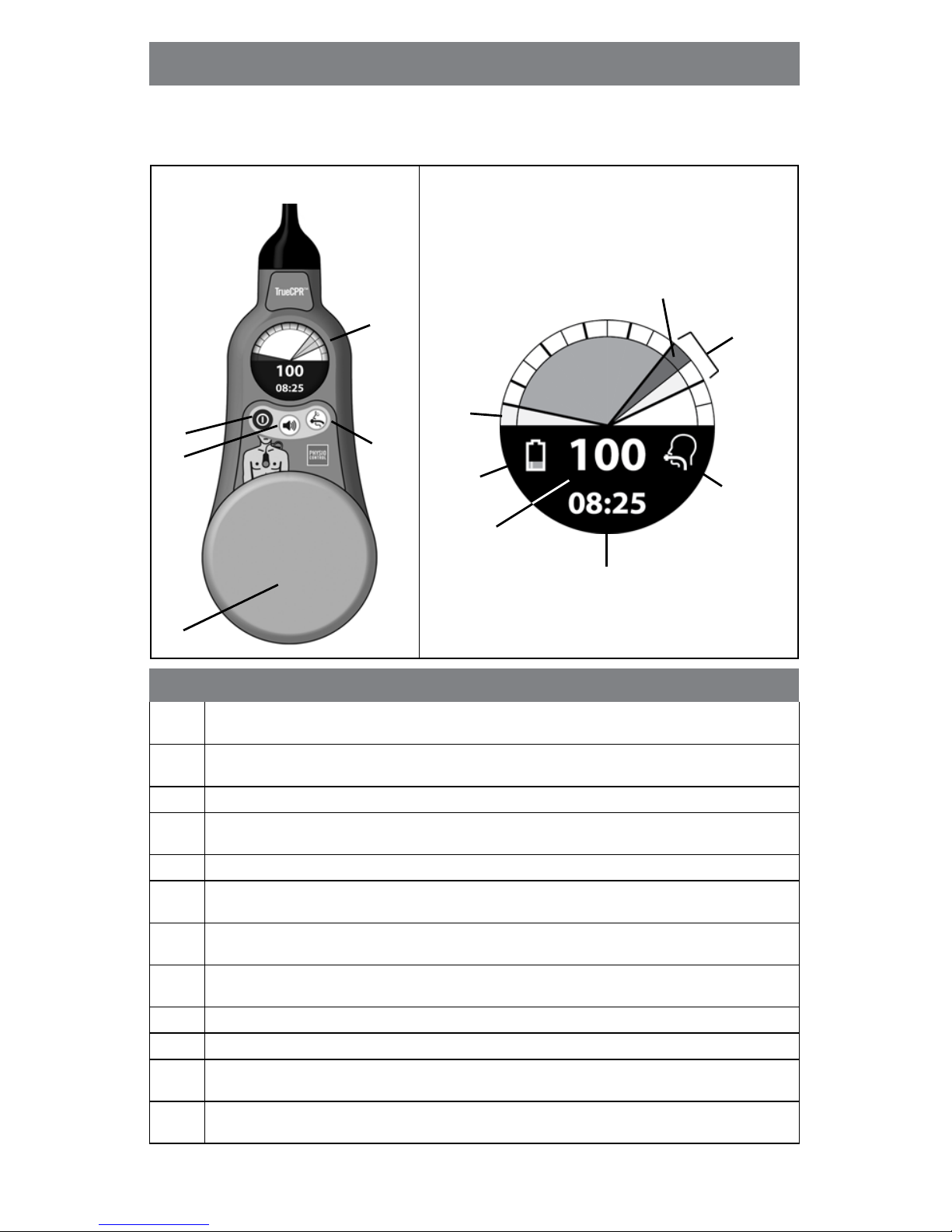
2 TrueCPR Device Instructions for Use
Safety Information
IMPORTANT! Check the battery readiness indicator regularly. If indicator is not
flashing, replace batteries immediately.
WARNINGS
POSSIBLE PATIENT INJURY:
• The TrueCPR device is not intended for use on children or infants. Only
use the device on patients who are 8 years of age or older.
POSSIBLE INEFFECTIVE CPR:
• After use, inspect device and cable for wear or damage. Remove from
service if damaged.
• Do not use the TrueCPR device over very large metal surfaces. Perform
CPR unaided instead.*
• The TrueCPR device may provide incorrect feedback if not positioned
properly.
• Check back pad and chest pad position after patient movement and
during transport.
• If patient is on a mattress, place a backboard beneath the patient
according to standard protocols. Then insert the device back pad
between the patient and backboard.
• If the device cannot be positioned correctly on the patient or fails to
operate, remove the device and perform CPR unaided.
POSSIBLE DEVICE FAILURE: Do not modify the TrueCPR device.
POSSIBLE ELECTRICAL INTERFERENCE WITH DEVICE
PERFORMANCE: Equipment operating in close proximity may emit strong
electromagnetic or radio frequency interference (RFI), which could affect the
performance of this device. Avoid operating the device near cauterizers,
diathermy equipment, or other portable and mobile RF communications
equipment. See “Separation Distances” on page 22 for recommended
distances of equipment. Contact Physio-Control Technical Support if
assistance is required.
POSSIBLE EXPLOSION HAZARD: Do not use this device in the presence of
flammable gases or anesthetics.
* The TrueCPR device has been designed for use on hospital beds,
stretchers, gurneys, and in ambulances. The device is compatible with
common patient-worn or implanted metal, such as jewelry, ICDs, or
pacemakers.