Intended Use
The A1CNow+® test provides quantitative
measurement of the percent of glycated
hemoglobin (%A1C) levels in capillary
(ngerstick) or venous whole blood samples. The
test is for professional use to monitor glycemic
control in people with diabetes.
Summary and Explanation
High levels of blood glucose result in over-
glycation of proteins throughout the body
including hemoglobin.1Glycation of hemoglobin
can occur at the amino termini of the alpha
and beta chains, as well as other sites with free
amino groups.1Hemoglobin A undergoes a slow
glycation with glucose that is dependent on the
time-average concentration of glucose over the
120-day life span of red blood cells.
The most prevalent and well-characterized
species of glycated hemoglobin A is A1C,
making up approximately 3% to 6% of total
hemoglobin in healthy individuals.1The
correlation of A1C and blood glucose levels
make it a useful method of monitoring long-term
blood glucose levels in people with diabetes.2
Previous studies, such as the Diabetes Control
and Complications Trial (DCCT) and the United
Kingdom Prospective Diabetes Study (UKPDS),
used glycated hemoglobin as a way to measure
overall glycemic control during the studies.
These studies, and others, have shown that
tight glycemic control is associated with fewer
diabetes-related complications (e.g., vision
problems, cardiovascular problems, and kidney
problems).3The National Glycohemoglobin
Standardization Program (NGSP) was
established to assure traceability of hemoglobin
A1C (A1C) results to the DCCT. Studies show
a direct relationship from %A1C to average
blood glucose (MBG) levels. For every 1%
change in A1C there is a change of about 30
mg/dl in MBG.4 The formula used to calculate
the mean (average) blood glucose levels from
the A1C levels is MBG = (31.7 x HbA1c) - 66.1.
To convert to mean plasma glucose (MPG) use5
MPG = MBG x 1.11.
A1C can be measured by a variety of
techniques, and over the past decade they have
expanded to include point-of-care assays. Point-
of-care assays are well suited to environments
such as healthcare providers’ ofces and clinics,
because they are generally easy to perform,
require no laboratory equipment, and provide
rapid turn-around-time from sampling to result.6
This immediate feedback of results enhances
provider/patient interaction and, therefore better
enables disease management.7
Principle of the Assay
Chek Diagnostics has developed an enabling
technology that incorporates microelectronics,
optics, and dry-reagent chemistry strips within
a reusable, self-contained, integrated hand
held monitor and a single-use test cartridge.
An unmeasured whole blood mixture (diluted) is
directly applied to the sample port, and results
are displayed in numeric form on the Monitor’s
liquid crystal display after 5 minutes. Having no
switches or buttons, the Monitor self-activates
upon insertion of the Test Cartridge. The
A1CNow+Monitor utilizes both immunoassay
and chemistry technology to measure A1C and
total hemoglobin, respectively. Upon the addition
of a diluted blood sample, blue microparticles
conjugated to anti-A1C antibodies migrate
along the reagent strips. The amount of blue
microparticles captured on the strips reects the
amount of A1C in the sample.
For the total hemoglobin (Hb) portion of the
test, the sample diluent converts Hb to met-
Hb. The intensity of met-Hb color measured
on the reagent strips is proportional to the
concentration of hemoglobin in the sample. Test
results are expressed as %A1C
(A1C ÷ total Hb x 100).
Calibration of the A1CNow+is performed with
a set of blood samples that have been value-
assigned by a National Glycohemoglobin
Standardization Program (NGSP) certied
laboratory using an NGSP reference method.
Total Hb calibration values for those samples
are obtained with a Total Hb analyzer (HemoCue
Hemoglobin Test System, HemoCue, Inc., Lake
Forest, CA). The calibration of the A1CNow+test
is thus traceable to the NGSP and to an NGSP
Certied Network reference method.
Specimen Collection and Storage
Note: No fasting or special diet is necessary.
Fingerstick
The A1CNow+test requires 5 microliters (μL)
of whole blood (1 large drop). Fingerstick blood
is obtained by standard techniques with any
lancing system. If alcohol is used for cleansing,
be sure the nger is completely dry before
lancing.
Venipuncture/Sample Collection for Venous
Draw
Venous blood should be collected into heparin
tubes (sodium or lithium, “green tops”). Blood
samples should be well-mixed and tested at
room temperature. Venous blood samples are
stable for up to 8 hours at room temperature and
up to 14 days in the refrigerator.
Warnings and Precautions
1. For in vitro diagnostic use only.
2. Carefully read and follow the Professional
Procedure Guide to ensure proper test
performance.
3. If refrigerated, bring sealed pouches and
Monitor to room temperature for one hour.
4. The A1CNow+Monitor and Test Cartridges
should not be used if either are cracked or
broken.
5. The Test Cartridges should not be used if the
foil pouch is damaged.
6. Add sample to A1CNow+Test Cartridge
within 2 minutes after pouch is opened.
7. All components of the A1CNow+system
are potentially biohazardous. Dispose of as
biohazardous waste.
8. The Dilution Buffer in the Sampler contains
ferricyanide in a buffered detergent solution.
Do Not Ingest. In case of contact with skin
or eyes, ush the area with large amounts of
water.
9. Do not reuse Test Cartridges or Sample
Dilution Kits.
Do not mix Monitors with Cartridges & Sample
Dilution Kits from different lots.
Kit Storage and Stability
• Pouched Test Cartridges, A1CNow+
Monitors, and Sample Dilution Kits may be
stored at room temperature (18-28°C) for up
to four months prior to use. Monitors, Test
Cartridges, and Dilution Kits stored at room
temperature must be thrown away if not used
within the four months.
• If the temperature label, placed on the
outside of every kit, is exposed to a
temperature in excess of 122°F/50°C, the
dot on the label will turn red and the product
should not be used.
• The Monitors, Test Cartridges, and Sample
Dilution Kits may be used until the expiration
date printed on the box and pouches when
stored refrigerated (2-8°C). Monitors, Test
Cartridges, and Sample Dilution Kits stored
in the refrigerator must be thrown away if not
used by the expiration date.
• Leave all components in their sealed
pouches until use. If refrigerated, ensure
pouches are at room temperature before use.
• Do not mix pouches and Monitors from
different lots.
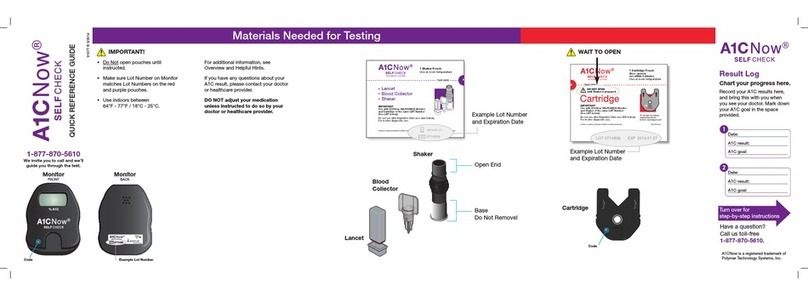
Package Components
• A1CNow+Monitor (1)
• A1CNow+Test Cartridges (10 or 20).
Each Test Cartridge includes the following
chemistries: antibody to HbA1c, antigen
conjugate that binds to the antibody, and
membranes.
• Sample Dilution Kit (10 or 20),
each containing:
º Sampler (1) containing 0.37 ml of buffered
detergent solution with ferricyanide
º Blood Collector (1)
• Product insert (1)
Materials Required but Not Supplied
• Fingerstick sample: lancet, or other blood
ngerstick collection device or,
• Venous Sample: Heparin (sodium or lithium
[“green top”]) preferred, venous collection
supplies.
• Gauze pad or cotton ball
• Bandage
• Liquid control solution. Contact Customer
Service (1-877-870-5610) for a list of liquid
controls that may be used.
Result Interpretation
Percent A1C monitors glucose control over
the last three months. About 50% of the A1C
result is from the past 30 days; about 25% is
from the past 30-60 days and about 25% is
from the past 60-120 days.1Depending on the
test methodology used, laboratory methods
show that the reference range of the A1C test
is approximately 4.0-6.5% A1C, and 6% to 9%
in people with well to moderately controlled
diabetes.1Levels can be as high as 20% in
people with poorly controlled diabetes.8The
American Diabetes Association’s (ADA’s) most
recent Clinical Practice Recommendation for
diabetes species a treatment goal for patients
in general of less than 7% with a treatment goal
for the individual patient of as close to normal
(less than 6%) as possible without signicant
hypoglycemia.9
Troubleshooting
See the table below for a description of
A1CNow+operating and error codes
(OR = Out of Range; QC = Quality Control,
E = Monitor Error)
MESSAGE DESCRIPTION AND RESOLUTION
OR 1 The blood sample may have too little
hemoglobin (less than 20% hematocrit), not
enough blood was collected, or the blood
was not well mixed inside the Sampler.*
You may wish to check hematocrit by
another method.
OR 2 The blood sample may have too much
hemoglobin (greater than 60% hemocrit),
or excess blood was collected.* You may
wish to check hemocrit by another method.
OR 3 The blood sample may have too little A1C,
or insufcient blood was collected.*
MESSAGE DESCRIPTION AND RESOLUTION
OR 4 The blood sample may have too much
A1C, or excess blood was collected.*
OR 5 The Monitor temperature is below 18°C
(64°F). Repeat the test at room temperature
(18-28°C).
OR 6 The Monitor temperature is above 28°C
(82°F). Repeat the test at room temperature
(18-28°C).
<4.0 The %A1C is less than 4%.
>13.0 The %A1C is greater than 13%.
QC 2 Occurs when you insert a Test Cartridge
that already has sample added to it. Do not
remove and reinsert a Test Cartridge after
adding sample.*
QC 6 Sample was added to Test Cartridge before
“SMPL” display. This counts down one test
on the Monitor. Remove and discard Test
Cartridge. To avoid this error, do not add
sample until the “WAIT” prompt clears and
“SMPL” appears.
QC 7 The Test Cartridge remained in the Monitor
without sample addition for 2 minutes after
“SMPL” prompt. This counts down one test
on the Monitor. Discard the Test Cartridge
and insert a fresh one when you are ready
to dispense the Sampler.
QC 30 to 33 The Monitor was unable to obtain a valid
initial reading. Be sure to remove the
Sampler within one second after
dispensing it into the sample port, and do
not disturb the Monitor while the test is
running.*
QC 50 to 51
QC 55 to 56
Insufcient sample was delivered to the
Test Cartridge. To avoid this error be sure
to fully insert the Blood Collector into the
Sampler and shake immediately.*
All other QC codes The quality control checks did not pass.
Call Customer Service toll free at
1-877-870-5610. The test will have to be
repeated with another Test Cartridge and
Sample Dilution Kit.
E1 to E99 The Monitor has a Fatal Error.
Call Customer Service toll-free at
1-877-870-5610.
*Carefully repeat the test using a new Test Cartridge and a new
Sample Dilution Kit.
Limitations
• This test is NOT for the screening or
diagnosis of diabetes.
• If the patient has high levels of Hemoglobin
F, Hemoglobin S, Hemoglobin C, or other
hemoglobin variants, the A1CNow system
may report incorrect results.
• Any cause of shortened red cell survival
(e.g., hemolytic anemia or other hemolytic
diseases, pregnancy, recent signicant blood
loss, etc.) will reduce exposure of red cells to
glucose. This results in a decrease in %A1C
values. Percent A1C results are not reliable
in patients with chronic blood loss and
consequent variable erythrocyte life span.
• Rheumatoid Factor in high amounts will
cause low results, or an error code. It is
recommended that A1C be re-checked by
alternate methodology such as boronate
afnity.
• This test is not a substitute for regular
healthcare provider visits and blood glucose
monitoring.
• As with any laboratory procedure, a large
discrepancy between clinical impression and
test results usually warrants investigation.
Controls
Each A1CNow+Monitor performs over 50
internal chemical and electronic quality
control checks, including potential hardware
and software errors (e.g. cartridge alignment,
programming), and potential reagent strip
errors (e.g. insufcient sample volume,
invalid calculations). The Monitor has been
programmed to report an error code if these
quality checks are not passed.
Quality control testing should be performed at
the following times:
1. With each new shipment.
2. With each new lot.
3. With each new operator.
4. Whenever problems (storage, operator,
instrument, or other) are identied.
5. To ensure that storage conditions have not
affected the product, run a control sample
before running a patient sample if the test kit
has been stored for more than a month and
it has been at least a month since the last
control testing.
The measured value should be within the
acceptable limits stated for the control material.
If the results obtained are outside the acceptable
limit, please review the procedure and re-test the
control material. If the measured value continues
to fall outside the acceptable limit, please refrain
from analyzing additional patient samples and
contact Customer Service (1-877-870-5610).
Good laboratory practices include a complete
quality control program. This entails proper
sample collection and handling practices,
ongoing training of testing personnel, ongoing
evaluation of control results, proper storage
of test kits, etc. A permanent record of control
results should be retained.
Performance
Expected Values (non-diabetic population)
The expected normal range for %A1C using
the A1CNow system was determined by testing
blood samples from 118 presumptively non-
diabetic individuals (fasting glucose levels <127
mg/dL) across three US sites. The population
included 33 males and 85 females, and an age
range from 19 to 76 with a mean age of 43. The
mean %A1C result was 5.2% ±0.71% (1 SD).
The 95% condence limits were 3.9% to 6.5%.
These values are similar to those reported in
the literature. Each laboratory should determine
its own reference range to conform to the
population being tested.
Linearity
Studies were performed to evaluate the linearity
of the A1CNow system across its dynamic
range. Clinical samples representing low
and high %A1C levels were identied, and
were mixed in various proportions into nine
preparations. These samples were tested in
replicates of at least ve (n = 5). The observed
results were compared to the expected results
and analyzed in terms of percent recovery.
The test is linear for %A1C levels between 4%
and 13%, and produces reliable results with
hematocrits between 20% and 60% packed cell
volume (PCV).
Interference Testing/Specicity
Studies were performed to assess the effect
of common test interferents, various common
over-the-counter therapeutic agents, and oral
antihyperglycemic agents commonly used
to treat Type II diabetes. Two levels of %A1C
(low and high, approximately 4% and 10%,
respectively) were tested. See table below.
INTERFERENT TEST CONCENTRATION
Bilirubin
(unconjugated)
20 mg/dL
Triglyceride 3000 mg/dL
Hemoglobin 500 mg/dL
Acetaminophen 80 μg/mL
Ascorbic acid 5 mg/dL
Ibuprofen 120 μg/mL
Acetylsalicylic acid 1 mg/mL
Glyburide
(glibenclamide)
240 ng/mL
Metformin (1.1-dimenthyl-
biguanide HCI)
25 μg/mL
The studies showed no effect from any of these
potential interferents at concentrations up to
approximately 5-times their normal levels or
therapeutic doses.
Studies showed no interference from modied
hemoglobins, including labile glycated
hemoglobin when tested at two levels of %A1C
(low and high, approximately 5% and 11%
respectively). The modied hemoglobins, and
the levels evaluated, were: labile hemoglobin
with 1400 mg/dL glucose, carbamylated
hemoglobin at a nal concentration of 5 mM
potassium cyanate, and acetylated hemoglobin
at a nal concentration of 14 mM acetylsalicylic
acid.
There were mixed results from the testing of
high levels of Hemoglobin F, Hemoglobin S,
and Hemoglobin C. Unreliable results may be
obtained from patients with elevated levels of
variant hemoglobins.
Precision
Precision testing was done under a specialized
protocol. Following this protocol, two whole
blood samples, one of approximately 6 %A1C
(low), and one of approximately 9 %A1C (high),
were tested over 20 days and four runs per day,
for a total of 80 assays per level. The overall
imprecision (including within-day and between-
day) was 3.00% CV at the low level and 4.02%
CV at the high level. This performance meets the
requirements of NGSP certication.
Accuracy
Accuracy studies were conducted with 189
diabetic and non-diabetic subjects across three
US sites. Fingerstick sampling was performed
on each subject for testing with A1CNow+,
and venous blood was collected from each
subject for comparative testing using an
NGSP-certied method. A1CNow+results were
compared to the NGSP reference results. The
A1C results ranged from 5.0 %A1C to 12.8
%A1C, with a mean of 7.3 %A1C (reference
results). Data analysis consisted of least squares
linear regression (x = reference results), bias
calculation, and Bland Altman limits. The data
are provided below.
A1CNow+Fingerstick Comparative Testing
(NGSP-certied method is the Tosoh A1c 2.2
Plus)
n 189 Bias at 6% A1C
(% difference)
5.89
(- 1.83%)
Slope 1.02 Bias at 7% A1C
(% difference)
6.91
(-1.29%)
y-
intercept
- 0.23 Bias at 9% A1C
(% difference)
8.95
(- 0.56%)
“r” 0.95 Avg. % diff. - 1.23%
The results showed that the accuracy of
A1CNow+, with ngerstick samples was, on
average, 99%. This means that, on average, a
true 7 %A1C could read approximately
6.9 %A1C. An individual A1CNow+result may
differ by as much as -1.0 %A1C to +0.8 %A1C
from the true result. This represents the 95%
condence limits of a Bland-Altman plot.
A1CNow+Venous Comparative Testing
(NGSP-Certied method is the Tosoh
A1c 2.2 Plus)
Venous blood was collected from 110 diabetic
subjects, and each sample was tested on one
of three different lots. Aliquots of the venous
samples were also tested by the NGSP-certied
method, providing comparative results. Data
analysis again consisted of least squares
linear regression (x = reference results), bias
calculation and Bland-Altman limits. The data
are provided below.
n 110 Bias at 6% A1C
(% difference)
5.95
(-0.8%)
Slope 1.03 Bias at 7% A1C
(% difference)
6.98
(-0.3%)
y-
intercept
-0.237 Bias at 8% A1C
(% difference)
8.01
(+0.1%)
“r” 0.97 Avg. % diff. -0.3%
The results showed that the accuracy with
venous sampling was, on average, 99.7%. An
individual result may differ by -0.8 %A1C to +0.7
%A1C from the true result. This represents the
95% condence limits of the Bland-Altman plot.
A1CNow+may be used with either ngerstick
(capillary) or venous (heparin-anticoagulated)
whole blood samples.
Expected Performance in Waived
Laboratories
Clinical studies were performed at three US sites
with over 180 untrained people (most with
diabetes). These study subjects read the
instructions and then performed one A1CNow+
test on themselves. A venous blood sample was
collected from each subject, and this sample
was tested by an NGSP-certied laboratory
method for %A1C. The two results were then
compared.
Untrained User A1CNow+and an
NGSP-certied method
(Tosoh A1c 2.2 Plus)
n 188 Bias at 6% A1C (%
difference)
6.02
(+ 0.33%)
Slope 0.99 Bias at 7% A1C (%
difference)
7.01 (+
0.14%)
y-
intercept
0.08 Bias at 9% A1C (%
difference)
8.99
(- 0.11%)
“r” 0.93 Avg. % diff. + 0.12%
The results showed that untrained users could
perform A1CNow+testing on themselves with
the same accuracy as trained individuals.
References
1. Buris, C.A., Ashwood, E.R. Tietz Textbook of Clinical
Chemistry, 3rd Edition, W.B. Saunders Co., 1999.
2. Nathan, D.M., et al. The clinical information value of the
glycosylated hemoglobin assay. N Engl J Med 1984; 310;
341-346.
3. The Diabetes Control and Complications Trial Research
Group. The effect of intensive treatment of diabetes on the
development and progression of longterm complication in
insulin-dependent diabetes melllitus. N Engl J Med 1993; 329;
977-986.
4. American Diabetes Association. Standards of medical care for
patients with diabetes mellitus. Diabetes Care.1999; 22 (suppl
1): S32–S41.
5. Fogh-Anderson, N., D’Orazio, P. Proposal for standardizing
direct-reading biosensors for blood glucose. Clin Chem 1998;
44(3); 655-659.
6. MLO Supplement. Point-of-Care Testing, 1992.
7. Cagliero, E., Levina, E.V., Nathan, D.M. Immediate feedback
of A1C levels improves glycemic control in type 1 and insulin-
treated type 2 diabetic patients. Diabetes Care 1999; 22(11):
1785-1789.
8. Goldstein, D.E., Little, R.R., Wiedmeyer, H.M., et al. Glycated
hemoglobin: Methodologies and clinical applications.
Clin Chem 1986; 32: B64-B70.
9. American Diabetes Association: Clinical Practice
Recommendations 2006. Diabetes Care, 2006; 29 (Suppl.1).
INTERNATIONAL SYMBOLS
MANUFACTURER
CONTAINS SUFFICIENT FOR
<N> TESTS
IN VITRO DIAGNOSTIC
MEDICAL DEVICE
AUTHORIZED REPRESENTATIVE
IN THE EUROPEAN
COMMUNITY
8°C
46°F
36°F
2°C
STORE REFRIGERATED
(2-8°C, 36-46°F)
CATALOG NUMBER
THIS PRODUCT FULFILS
THE REQUIREMENTS OF
DIRECTIVE 98/79/EC ON IN
VITRO DIAGNOSTIC MEDICAL
DEVICES.
CONSULT INSTRUCTIONS
FOR USE
IMPORTANT
USE BY
This test is WAIVED under the Clinical Laboratory Improvement Amendments of 1988 (CLIA).
If a laboratory modies the test instructions, the test will no longer be considered waived.
PROFESSIONAL-USE PRODUCT INSERT
MDSS GmbH
Schiffgraben 41
30175 Hannover, Germany
Polymer Technology Systems, Inc.
7736 Zionsville Road
Indianapolis, IN 46268 USA
1-877-870-5610