There is a break of 30 seconds between the measurements. A count
down indicates the remaining time.The individual results are not
displayed. Your blood pressure will only be displayed after all
measurements are taken. Do not remove the cuff between
measurements.
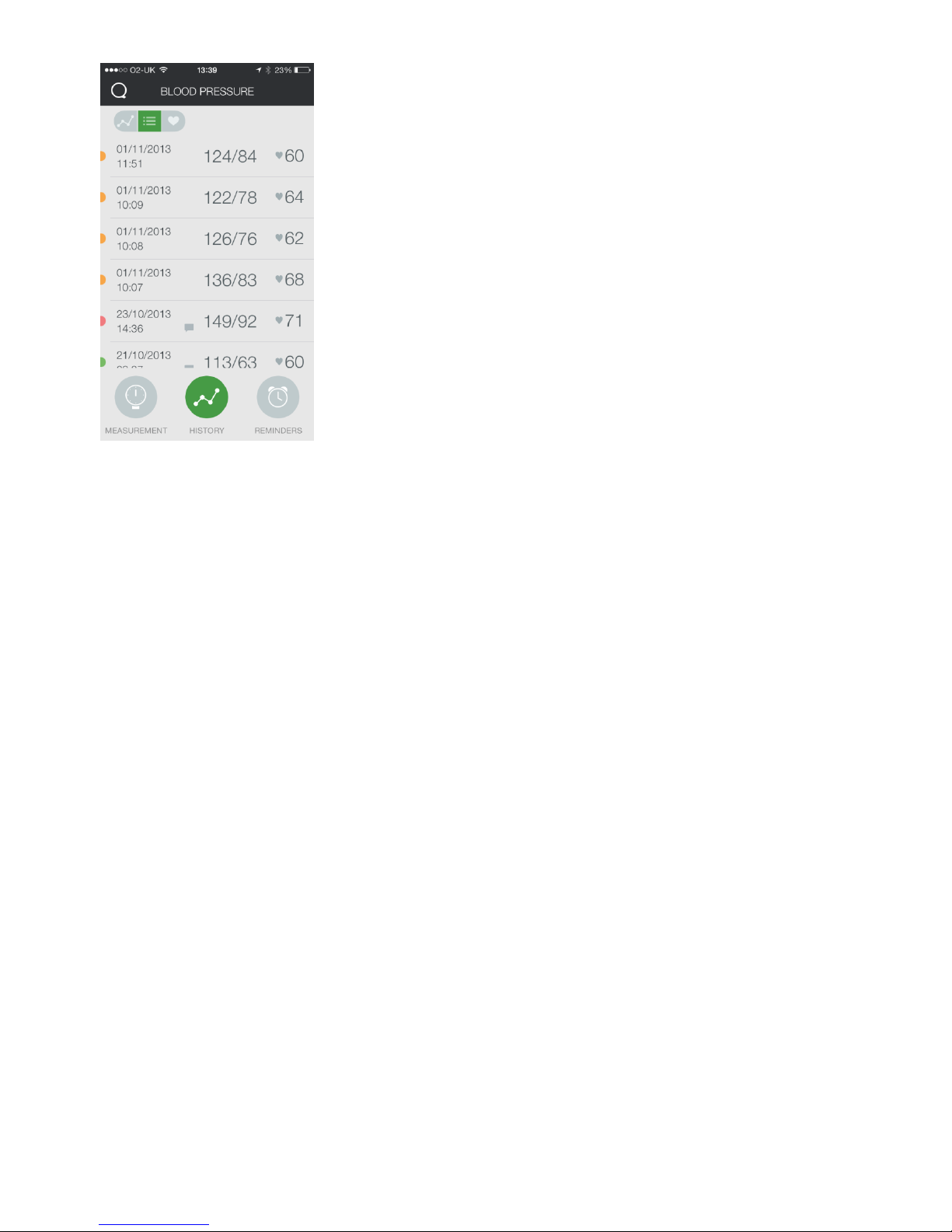
Visualizing your historical blood pressure data
Press the History button on the Blood Pressure page to visualize your
historical blood pressure and heart rate data in a table or chart format.
Important Facts about Blood Pressure and Self-Measurement
QardioArm measures your blood pressure. Blood pressure is the
pressure of the blood flowing in the arteries generated by the pumping
of the heart. Two values, the systolic (upper) value and the diastolic
(lower) value, are always measured.
QardioArm also measures your pulse rate. Pulse rate is the number of
times the heart beats in a minute.
High blood pressure, especially when permanent or recurrent, can
negatively affect your health and must be treated by your doctor.
Always discuss your measurement readings with your doctor and tell him/her if you have noticed
anything unusual or feel unsure. Never rely on a single blood pressure reading.
There are several potential causes of high blood pressure. Your doctor will explain them in more detail
and offer treatment where appropriate. Besides medication, weight loss and exercise can also help
lowering your blood pressure.
You should never alter the dosages of any medications prescribed by your doctor.
Blood pressure is subject to wide fluctuations throughout the day, depending on various potential
factors, including physical exertion and condition. You should routinely take your measurements in quiet
conditions when you feel relaxed. Ideally, you should take two readings every time (in the morning and
in the evening) or as prescribed by your doctor.
Deviations between measurements taken by your doctor or in the pharmacy and those taken at home
are quite normal, as these situations are completely different.
It is recommended to have at least 30 seconds in between measurements.
If you are pregnant, consult your healthcare provider before use. Monitor your blood pressure regularly
throughout pregnancy as it can change drastically during this time. When you detect unusual high
readings during pregnancy, you should measure again after at least four hours. If the reading is still too
high, consult your doctor or obstetrician.
Physical activity including eating, drinking, and smoking as well as excitement, stress, and many other
factors influence blood pressure results.
How to evaluate your blood pressure?
The World Health Organization (WHO) has created the following guide for assessing high blood pressure
(without regard to age or gender). It is important to note that various factors (e.g. diabetes, obesity,
smoking, etc.) also need to be considered. Consult with your physician for an accurate assessment and
diagnosis of your health condition.
This chart is not intended to provide a basis for any type of diagnosis or emergency assessment; this
chart only depicts different classifications of blood pressure. Consult your physician for an interpretation
and diagnosis based on your blood pressure results.