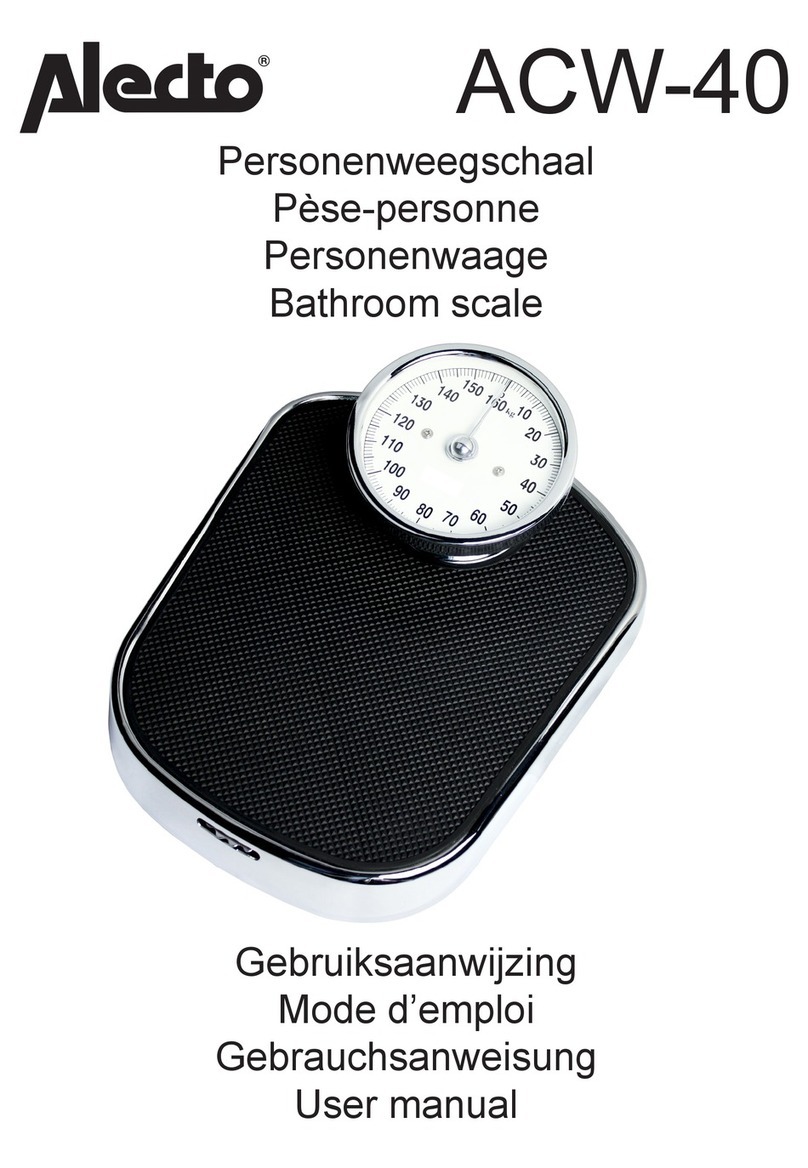
Sartorius 2405 User guide
Other Sartorius Scale manuals

Sartorius
Sartorius Master Pro Series User manual

Sartorius
Sartorius Cubis MCA Specification sheet

Sartorius
Sartorius Element Series User manual

Sartorius
Sartorius Master series User manual

Sartorius
Sartorius M3P User manual

Sartorius
Sartorius Combics CAW1P User manual

Sartorius
Sartorius CW3P User manual

Sartorius
Sartorius SIWABBP User manual

Sartorius
Sartorius BL310 User manual

Sartorius
Sartorius Cubis MCA10202S Series User manual

Sartorius
Sartorius Extend Series User manual
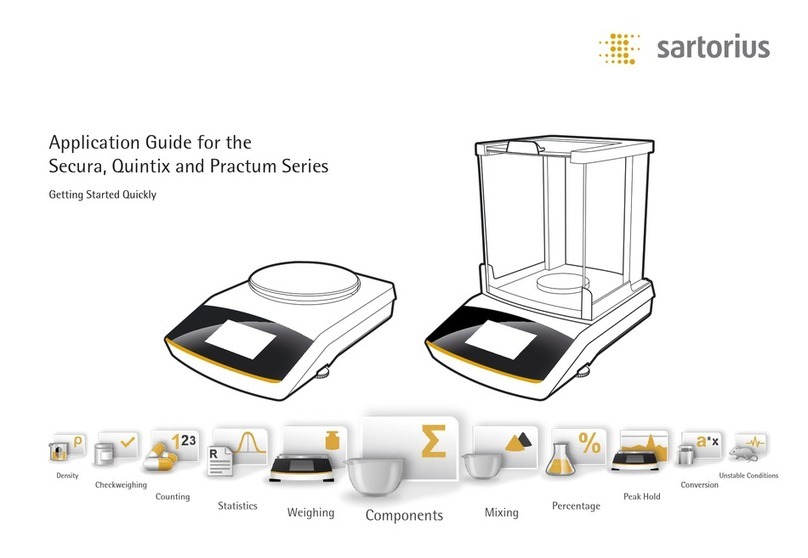
Sartorius
Sartorius secura series User guide

Sartorius
Sartorius FC User manual

Sartorius
Sartorius PMA.HD User manual

Sartorius
Sartorius Micro MC Series User manual

Sartorius
Sartorius Master series User manual

Sartorius
Sartorius TopMix2.Touch TM02-X User manual

Sartorius
Sartorius PMA.Evolution EVO1Y1 User manual

Sartorius
Sartorius R 160 P User manual

Sartorius
Sartorius talent User manual