.
Contents
1. FOREWORD.................................................................................................................................................................................................... 4
1.1. SYMBOLS USED........................................................................................................................................................................................4
1.2. SYMBOLS ON THE DEVICE ......................................................................................................................................................................4
1.3. RELEVANT EUROPEAN DIRECTIVES ......................................................................................................................................................4
1.4. CLASSIFICATION.......................................................................................................................................................................................4
1.5. INTENDED USE .........................................................................................................................................................................................5
1.5.1. IMPORTANT NOTES.........................................................................................................................................................................5
1.6. GENERAL WARNINGS...............................................................................................................................................................................5
1.7. RESIDUAL RISKS.......................................................................................................................................................................................6
1.8. INFORMATION ON MITIGATION OF RESIDUAL RISKS ...........................................................................................................................6
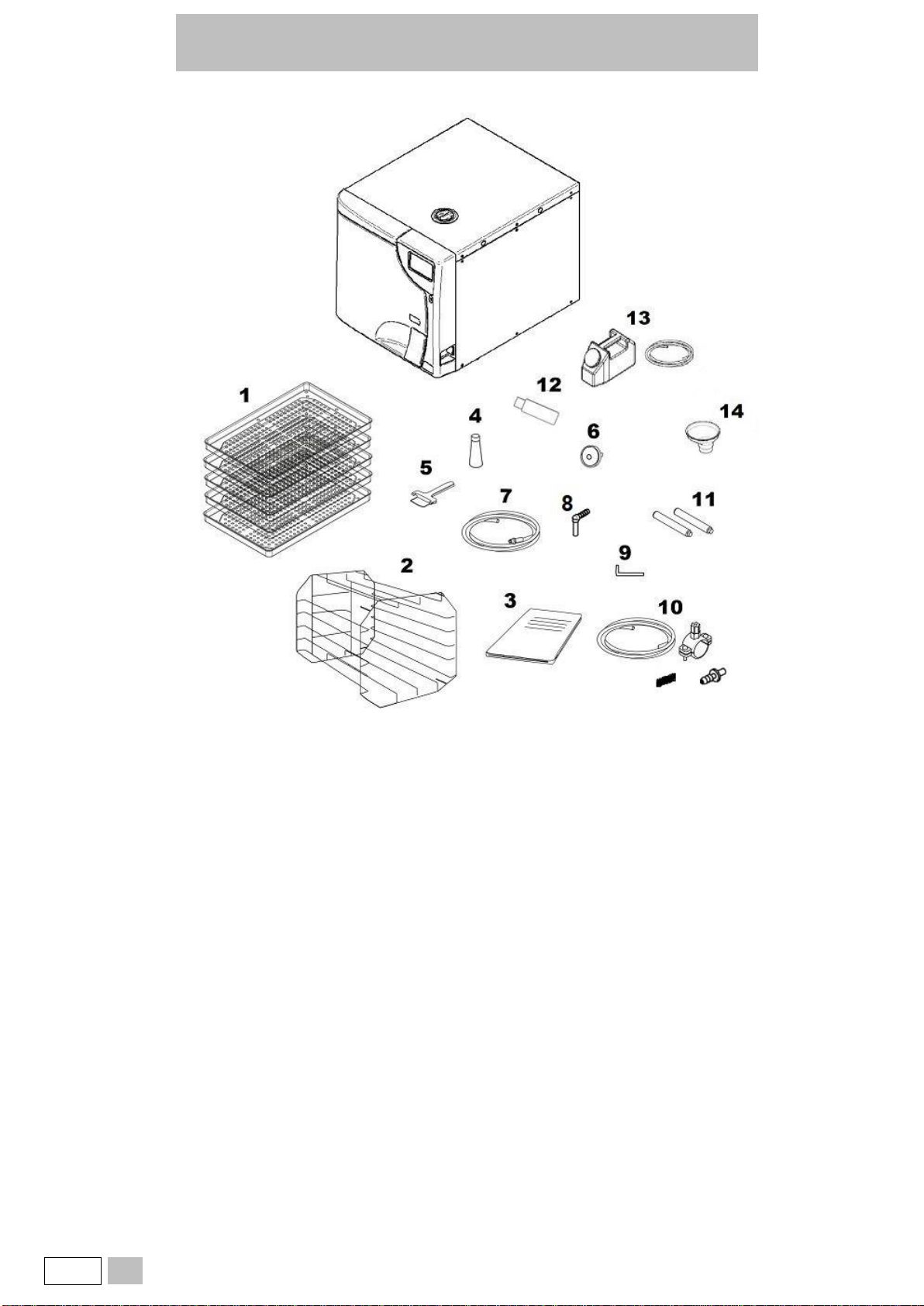
2. PACKAGE CONTENT..................................................................................................................................................................................... 7
2.1. DIMENSION AND WEIGHT ........................................................................................................................................................................7
2.2. DESCRIPTION OF THE CONTENT............................................................................................................................................................8
2.3. PRODUCT HANDLING...............................................................................................................................................................................9
2.4. CONDITIONS FOR STORAGE AND TRANSPORT....................................................................................................................................9
3. GENERAL DESCRIPTION - PRODUCT PRESENTATION............................................................................................................................ 10
3.1. GENERAL CHARACTERISTICS...............................................................................................................................................................10
3.2. TECHNICAL SPECIFICATIONS ...............................................................................................................................................................11
3.2.1. SUMMARY TABLE...........................................................................................................................................................................11
3.3. SAFETY DEVICES....................................................................................................................................................................................13
3.4. WATER SUPPLY CHARACTERISTICS....................................................................................................................................................14
3.5. FRONT .....................................................................................................................................................................................................15
3.6. REAR........................................................................................................................................................................................................16
3.7. LCD ICONS ..............................................................................................................................................................................................17
3.8. EXAMPLE OF WORKING CYCLE ............................................................................................................................................................18
4. SETTING UP THE DEVICE............................................................................................................................................................................ 19
4.1. OVERALL DIMENSIONS..........................................................................................................................................................................20
4.2. COMPARTMENT DIMENSIONS FOR BUILT-IN INSTALLATION.............................................................................................................21
4.3. GENERAL PRECAUTIONS FOR INSTALLATION....................................................................................................................................21
4.4. POWER SUPPLY .....................................................................................................................................................................................21
4.5. ELECTRICAL CONNECTIONS.................................................................................................................................................................22
4.6. DIRECT CONNECTION TO A CENTRALISED DRAINING POINT ...........................................................................................................22
5. FIRST START-UP.......................................................................................................................................................................................... 23
5.1. STARTING................................................................................................................................................................................................23
5.2. MAIN MENU .............................................................................................................................................................................................25
5.3. FILLING DEMINERALISED / DISTILLED WATER ....................................................................................................................................25
5.3.1. MANUAL FILLING............................................................................................................................................................................25
5.3.2. AUTOMATIC FILLING......................................................................................................................................................................25
6. CONFIGURATION......................................................................................................................................................................................... 26
6.1. SETTINGS................................................................................................................................................................................................26
6.1.1. LANGUAGE.....................................................................................................................................................................................26
6.1.2. DATE AND TIME..............................................................................................................................................................................27
6.1.3. REMINDER......................................................................................................................................................................................27
6.1.4. USERS.............................................................................................................................................................................................28
6.1.4.1. USERS LIST....................................................................................................................................................................................29
6.1.5. PREFERENCES ..............................................................................................................................................................................30
6.1.5.1. UNIT OF MEASUREMENT ..............................................................................................................................................................31
6.1.5.2. DISPLAY..........................................................................................................................................................................................31
6.1.5.3. WATER FILLING..............................................................................................................................................................................32
6.1.5.4. PREHEATING..................................................................................................................................................................................33
6.1.6. SERVICE .........................................................................................................................................................................................33
7. PREPARATION OF THE MATERIAL ............................................................................................................................................................ 34
7.1. TREATING THE MATERIAL BEFORE STERILISATION...........................................................................................................................34
7.2. ARRANGING THE LOAD..........................................................................................................................................................................35
7.3. POSITIONING AND USE OF TRAY HOLDER SUPPORT........................................................................................................................37
8. STERILISATION CYCLES............................................................................................................................................................................. 38
8.1. EXTRA DRYING.......................................................................................................................................................................................39
8.2. DELAY START..........................................................................................................................................................................................40
8.3. EXECUTION OF THE CYCLE...................................................................................................................................................................41
8.4. CYCLE OUTCOME...................................................................................................................................................................................41
8.5. DOOR OPENING AT THE END OF THE CYCLE......................................................................................................................................41
8.6. USER-DEFINED CYCLE...........................................................................................................................................................................42
9. MATERIAL STORAGE .................................................................................................................................................................................. 43
10. TEST PROGRAMS........................................................................................................................................................................................ 44
10.1. HELIX / B&D TEST CYCLE.......................................................................................................................................................................44