SootheNeb NBL100 User manual

311-7001200-035
Version 4.0 2017/11
1. Mouthpiece and mask are intended for use by one user. DO NOT SHARE.
2. Accessories are consumable parts, e.g. mesh cap. Clean the accessories after each use and
replace with new accessories when they become dirty and obstructed.
3. Mask and AC Adapter may be provided separately, please contact your local dealer for
purchase, or contact our customer service line for more information.
NOTE
Do not wet the connector and connector socket (refer to Description of Part 3 & Part 4).
Use the equipment only for its intended use as described in the operating instructions. Do not use
attachments not recommended by the manufacturer. Use the AC Adapter (Input: AC, 100-240V
& Output: DC, 6V) provided by ForaCare, Inc.
Do not use the device under conditions of inter-hospital transport.This device is not intended for
transport use.
Use the equipment with medications only under the instruction of your physician.
Do not use the equipment if it has any damaged parts, or it has fallen into water.
Prior to using the equipment for the first time, or after storing it for an extended period, be sure to
clean all necessary parts as described in the cleaning instructions.
Do not use while bathing.
The unit should not be left unattended while plugged in or turned on.
Keep the unit out of reach of small unsupervised children. The small parts detached from the device
may be a choking hazard if inhaled or swallowed.
To prevent any dangers or hazards, please do not try to modify the device in any way.
Do not place the device in liquid, nor put it where it could fall into liquid. If the device becomes
wet, unplug the device before touching it.
Do not use the device if it is not working properly, or if it has suffered any damage.
Avoid dropping, smashing, hitting, or tapping the nebulizer unit or the mesh cap.
Please clean the nebulizer accessories after every use, or after storing for a period of time.
Do not expose the nebulizer to direct sunlight, high temperatures or humidity.
Performance information provided by the manufacturer in accordance with the standard EN
13544-1:2007 may not apply to drugs supplied in suspension or high viscosity form. Please consult
the drug supplier for such information.
IMPORTANT INFORMATION ABOUT INHALATION THERAPY
SAFETY PRECAUTIONS
The device is ideally suitable for inhalation at home or when traveling.
Carry out inhalation in a quiet and relaxed state, and inhale slowly and deeply, so that the medication
can penetrate to the fine, deep bronchial tubes. Exhale normally.
Ensure that all parts are clean and dry.
Before starting treatment, talk to your physician about the duration, dosage and frequency of use
of the nebulizer.
The indicator light will turn green when the device is working normally.
The device automatically turns off when idle for 15 minutes.
2. Attach the mesh
cap and screw it
tightly in clockwise
direction.
1. Press and release the
ON/OFF button.The unit
turns on and delivers
prescribed medication
continuously for up to 15
minutes.
4. Attach the mask or the mouthpiece to the adapter.
5. Connect the AC adapter or go to step 6 if the batteries are
already installed.
6. Press the ON/OFF button to begin treatment according to
the physician’s instructions. See "Delivery Mode" for details
on automatic and manual deliveries.
7.To end the treatment, press the ON/OFF button to turn off.
OPERATING INSTRUCTIONS
Important information before use. These operating instructions provide important information to
help you use the device properly.
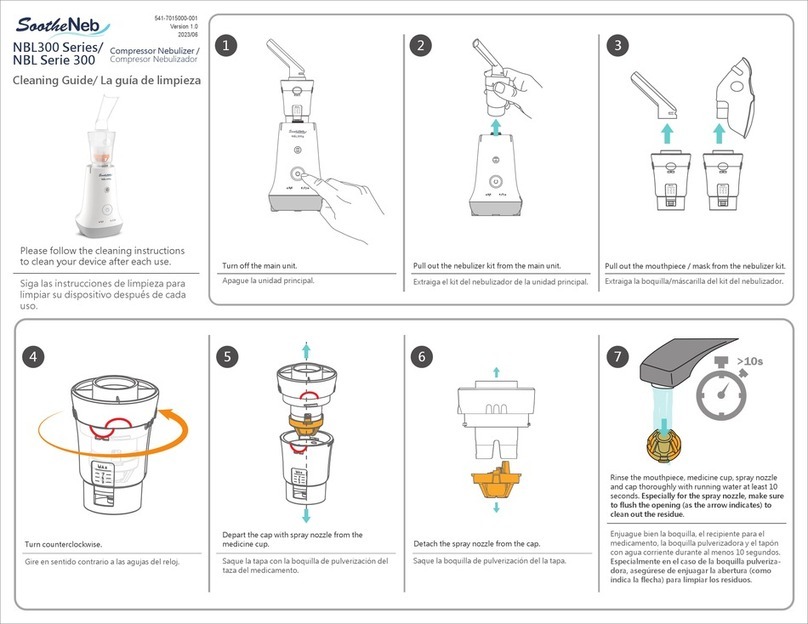
Unit Assembly and Usage
DELIVERY MODE
CLEANING AND MAINTENANCE
This system provides you with two delivery modes: automatic or manual.
Cleaning of Nebulizer Accessories
Please clean your device accessories after each use.
Prior to cleaning, make sure you have some
distilled water
on hand and ensure that the device has
completed the last medicine delivery cycle and is completely turned off.
* AUTOMATIC AEROSOL DELIVERY
FEATURES
CONTENTS INCLUDED & DESCRIPTIONS
INTENDED USE
Delivers aerosol automatically or manually
Battery powered or AC adapter connection (optional)
Small nebulization particle size – approx. 5 microns
Convenient design – compact and lightweight. Ideal for traveling.
Short treatment time – with high nebulization rate
Dear SootheNeb NBL100 Owner:
Thank you for purchasing the SootheNeb NBL100, before using this product, please read the following
contents thoroughly and carefully.
If you have other questions regarding this product, please contact the place of purchase or call our
customer service line at 888.307.8188.
SootheNeb NBL100 is an ultrasonic (vibrating mesh) nebulizer system designed to aerosolize
physician-prescribed solution for inhalation by the patient, except for Pentamidine.The SootheNeb
NBL100 is intended for use by patients in a home care environment.
Cautions: Federal law restricts this device to only be sold by or on the order of a physician.
Before you start to use your SootheNeb NBL100,
please check that the following contents are included
in the package and not broken. The use of other
contents not listed may constitute a hazard.
Mesh Nebulizer Operating Instructions
NBL100
1.
Unit Lid
Mesh Cap
Connector
Connector Socket
Indicator
2.
3.
5.
4.
6.
Mask or Mouthpiece Adapter
7.
8.
ON/OFF Button
10.
Medication Cup
9.
Battery Cover
11.
13.
2“AA”Alkaline Batteries
14.
15.
Mouthpiece
AC Adapter
Mask
OPTIONAL PARTS**
INCLUDED PARTS
12.
2.
1.
4.
3.
5.
6.
7.
8.
10.
9.
11.
15.14.
13.
12.
Nebulizer Unit
AC Adapter Jack
1. Pour the prescribed
medication slowly into
the medication cup.
The maximum capacity
is 5 ml.
3. Attach the mask or
mouthpiece adapter.
2. Press the ON/OFF
button to switch off
the device and
terminate the
treatment.
1. Press and hold the
ON/OFF button for 2
seconds or longer to
begin medication
delivery.
1. Remove the Mask or Mouthpiece and remove the Mask/Mouthpiece adapter from the Main Unit.
Rinse thoroughly and set aside to dry. Then remove the Mesh Cap counterclockwise from the
Medication Cup and keep the medication cup attached.
To deliver medication manually, press the ON/OFF button intermittently to accompany your breathing
rate.
* MANUAL AEROSOL DELIVERY
Repeat step 1 and 2 for additional medication deliveries.
2. Release the ON/OFF
button to stop
medication delivery.

1. The above-named accessories are consumable items. Please replace with new
accessories when they become dirty and obstructed. Do not share the accessories with others.
2. Use only the accessories and parts from ForaCare , Inc.
NOTE
Note: If vapor is slow, remove mesh cap and
shake off the residual water inside the mesh cap.
Be careful
NOT
to touch the mesh itself.
NOTE
Distributed by ForaCare, Inc.
893 Patriot Dr., Suite D
Moorpark, CA 93021 USA
Products made in Taiwan
Toll Free: 1-888-307-8188 (8:30am-5:00pm PST, Monday-Friday)
For assistance outside of these hours, please contact your healthcare professional.
Read instructions before use.
Maintenance for Storage
1. Cover the mesh cap with unit lid (refer to Description of Part #1) to prevent the accumulation of dust.
2. Always handle your nebulizer with care.
3. Disconnect the power plug from the wall outlet when not in use.
4. Keep your nebulizer out of children’s reach.
5. If you store your nebulizer, try to keep it in the following environmental ranges:
a) Temperature: -13°F to 158°F (-25°C to 70°C)
b) Relative humidity: 10% to 95%
6. If possible, store your nebulizer in a well-ventilated room.
7. Remove the batteries when the nebulizer is not in use for an extended period of time.
1. Use the AC Adapter (Input: AC, 100-240V & Output: DC, 6V) provided by ForaCare, Inc. DO
NOT use any other AC Adapters.
2. Discard the used batteries according to your local regulations.
NOTE
To replace the battery, make sure that the nebulizer is turned off.
1. Press the buckle on the battery cover and lift up to remove the cover.
2. Remove the used batteries. Insert the new ones. Correctly align the polarities (+ and -) with battery
indication marks on the device.
3. Close the battery cover by pressing down firmly until a click is heard.
Battery Replacement
Replace the batteries when the light indicator is red.
Disposal
Dispose of the device, components and optional accessories according to your local regulations.
Particle Size Delivery Test according to EN13544:2007
The mass median aerodynamic diameter (MMAD), geometric standard deviation (GSD), respirable
fraction (%, 0.5-5 µm), and particle size distribution of the particles generated from the SootheNeb
NBL100 are determined by laboratory testing conducted with a cascade impactor method according to
the European Standard for nebulizers (EN 13544-1:2007).
Three different drugs were used in the testing to represent three different drug classes: Ipratropium
bromide (anti-cholinergic bronchodilator), Ventolin (as known as albuterol, a beta-agonist bronchodila-
tor) and Cromolyn sodium (anti-inflammatory).The dose of each drug that used at the beginning of the
cascade impactor testing was:
The test had involved three runs each of three separate device samples tested with three classes of
drugs.The durations of each sample collecting by cascade impactor is about 1-3mins to allow for
maximum deposit on each stage without overloading. The result summary of the test is shown in
Table 1, CV (%) of repeatability was less than≦5%.
ADDITIONAL INFORMATION
2. Pour out any remaining medication.
Ipratropium: 500ug/2mL Ventolin: 5000ug/2.5mL Cromolyn: 8000ug/2mL
The test result of device sample I with three classes of drugs each in three runs is provided in Table 2
shown more information, including the total dose delivered, respirable mass (0.5-5 µm), course
particles fraction (>4.7 microns) (%), fine particles fraction (<4.7 microns) (%), and extra-fine particles
fraction (<1 micron) (%).
* I.B. = Ipratropium bromide
* V.= Ventolin
* C.S.= Cromolyn sodium
* MMAD (µm) = mass-median aerosol diameter, the diameter above and below which lies
50 % of the mass of the particles.
* GSD= Geometric standard deviation
* Respirable fraction (%, 0.5-5 µm) = Respirable mass (µg)/ Particle mass collected by the
cascade impactor (µg) x 100%
Table 2 Summary of the test results for
device sample I.
The ANOVA statistical analysis of the data from the performance test has been applied in the data
analysis. Results show that there is no significant difference (p > 0.05) between the mass size
distributions of the medicine particles generated by the proposed device and a legally marketed
device (predicate device).
In conclusion, the performance test results support the specifications of SootheNeb NBL100, which
has mass-median aerosol diameter (MMAD) less than 5µm and respirable fraction range from 60% to
80% depends on the drug type.
Symptom Probable Cause Solution
The indicator turns red.
The device does not work
when the ON/OFF button is
pressed.
The indicator is green but
the device does not produce
mists or produces small
amounts of mists.
The batteries do not have enough
power to perform.
The mesh cap is covered with dust. Clean the mesh cap.
If the situation happens again, please
replace with a new mesh cap.
No power. Check the batteries.
The mesh cap and medication cup are
not assembled properly.
The texture of medicine may be thick.
Check connection between the AC
adapter and the nebulizer.
Re-assemble the mesh cap and
medication cup and twist them well.
Gently shake the medicine well.
No medicine is left. Add the appropriate amount of
medicine prescribed by your physician
to the medication cup.
Mesh is clogged or dirty. Repeat the cleaning procedures step 1
to 7.
Replace the batteries immediately.
Features Device sample I.
Total output
mass (µg)
Respirable
Mass
(µg, 0.5-5 µm)
Coarse particle
Fraction
(%)(> 4.7 µm)
Ipratropium bromide – 438.94µg
Ventolin – 889.56µg
Cromolyn sodium – 911.49µg
Ipratropium bromide – 11.01%
Ventolin – 28.41%
Cromolyn sodium – 27.93%
Fine particle
Fraction (%)
(< 4.7 µm)
Ipratropium bromide – 88.99%
Ventolin – 71.59%
Cromolyn sodium – 72.07%
Ultra-fine
particle Fraction
(%)(< 1 µm)
Ipratropium bromide – 18.58%
Ventolin – 12.58%
Cromolyn sodium – 17.95%
Ipratropium bromide – 86.21µg
Ventolin – 224.3µg
Cromolyn sodium – 160.51µg
TROUBLESHOOTING
The device has been certified to meet the electrical and safety requirements of: IEC 60601-1, IEC 60601-1-2.
Cascade Impactor Testing*:
*The test method and results are shown in“Additional Information”.
Please contact your dealer for assistance with any other difficulties.
NOTE
Drug Name Ipratropium bromide
1.89µm
2.05
85.6%
2.87µm
2.4
74.6%
2.7µm
2.72
74.7%
Cromolyn sodiumVentolin
MMAD (micron)
GSD(geometric standard deviation)
Respirable fraction (% mass 0.5- 5µm)
Features
SPECIFICATIONS
Model no.: SootheNeb NBL100
Dimension & Weight: 53(D)mm x 103(H)mm, 83g
Power Source: 2 x AA alkaline batteries
Input: 100~240V, AC
Output: 6V, 1A, DC
Operating conditions: 41°F to 104°F (5°C to 40°C), 15% to 93% relative humidity
Storage conditions: -13°F to 158°F (-25°C to 70°C), 10% to 95% relative humidity
Power consumption: 1.2W
Nebulization rate: 0.25 ml/min
Medication capacity: 5 ml
MMAD: 5 microns
Features Mean C.V. (%)≦5%.
MMAD (µm)
Geometric
Std. Dev.
(GSD)
Respirable
Fraction
(%, 0.5-5 µm)
Ipratropium bromide – 1.89µm
Ventolin – 2.87µm
Cromolyn sodium – 2.7µm
I.B. – 1.61
V. – 1.25
C.S. – 1.49
I.B. – 2.69
V. – 2.05
C.S. – 1.52
I.B. – 0.43
V. – 0.72
C.S. – 0.41
Ipratropium bromide – 85.6%
Ventolin – 74.6%
Cromolyn sodium – 74.7%
Ipratropium bromide – 2.05
Ventolin– 2.4
Cromolyn sodium – 2.72
Table 1 Results of the repeatability test for
SootheNeb NBL100.
1. Use cotton cloth or paper towel to wipe clean the nebulizer unit (refer to Description of part 6)
2. Let it air dry on a clean paper towel.
Cleaning of Nebulizer Unit
3. Submerge
ONLY
the
Mesh Cap (NOT the medication cup)
in
distilled water
for about 30 seconds.
Then, carefully dry with a clean paper towel.
4. Add a small amount (approximately 1 ml) of
distilled water
into the Medication Cup.
5.Twist the mesh cap back on to the medication cup then
press the on button to nebulize the distilled water for
1 to 2 minutes to remove residual medication.
6.Turn off the device after it's done then remove the medication cap to air DRY on a clean paper towel.
7. Once all components are DRY, assemble device and store in a clean environment.
Distilled
Water
Residual Water
WARNING
Do not wet the medication cup connector and
the connector socket.
Other SootheNeb Respiratory Product manuals