ii rev
5.3.1 Positioning devices .................................................................................. 37
5.3.2 General instructions ................................................................................. 38
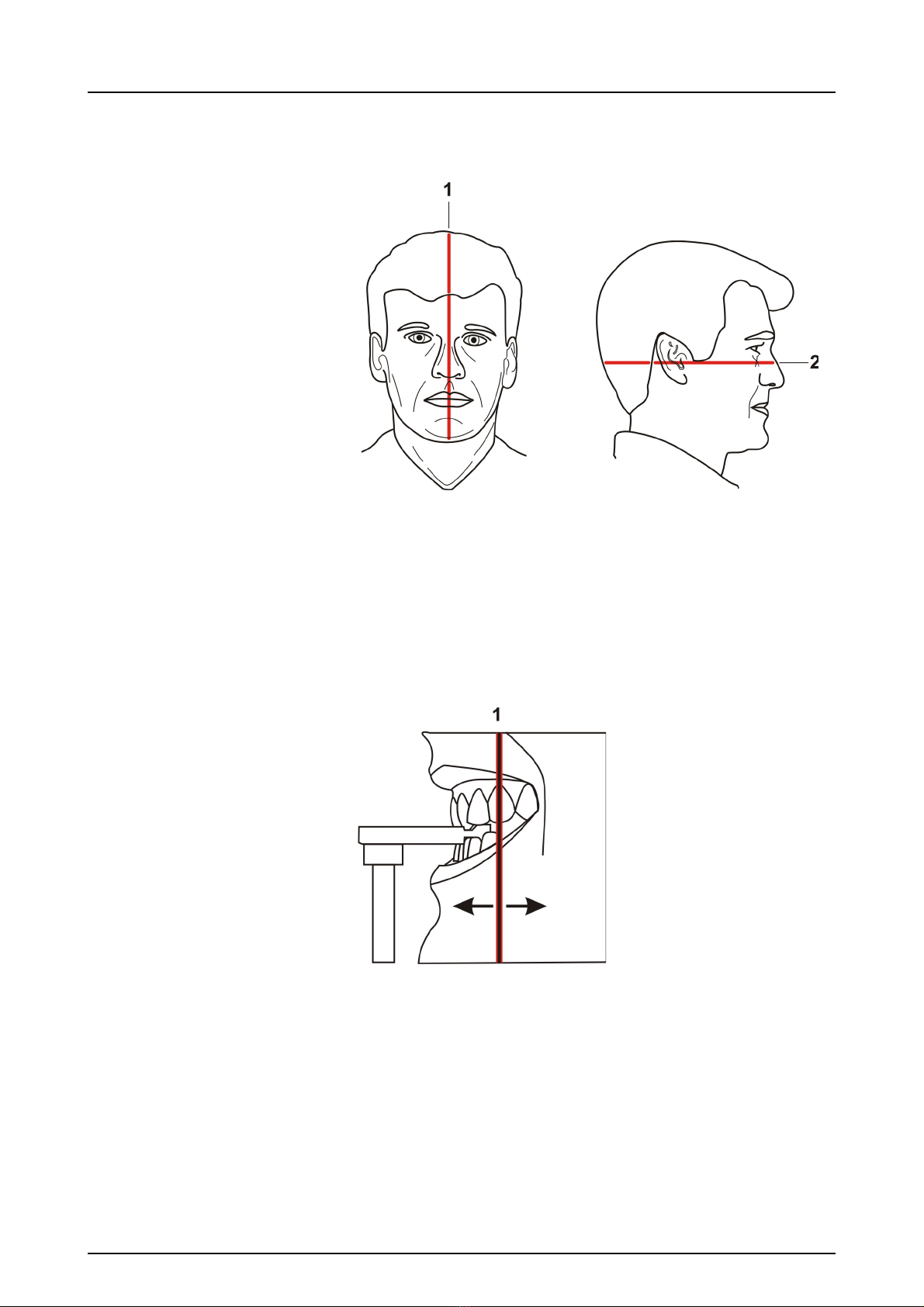
5.3.3 Patient positioning.................................................................................... 39
5.3.3.1 Panoramic exposure.................................................................. 39
5.3.3.2 TMJ exposure............................................................................ 43
5.3.3.3 Maxillary Sinus exposure........................................................... 45
5.3.3.4 Taking a panoramic exposure ................................................... 47
5.4 Cephalometric exposures ................................................................................... 48
5.4.1 General instructions ................................................................................. 48
5.4.2 Patient positioning.................................................................................... 50
5.4.2.1 Full width and reduced width projection..................................... 50
5.4.2.2 PA projection ............................................................................. 52
5.4.2.3 Reverse towne projection .......................................................... 54
5.4.2.4 Waters view ............................................................................... 55
5.4.2.5 Carpus program (optional)
(Not available in USA and Canada)........................................... 57
5.4.2.6 Taking a cephalometric exposure.............................................. 58
5.5 3D exposures ...................................................................................................... 60
5.5.1 Positioning devices .................................................................................. 60
5.5.2 General instructions ................................................................................. 60
5.5.3 Taking a Scout image .............................................................................. 63
5.5.4 Taking a 3D image................................................................................... 65
5.5.5 Stone model and radiographic guide scan............................................... 66
5.6 Warnings and error messages ............................................................................ 68
5.6.1 Acknowledging errors............................................................................... 68
5.6.2 Image transfer errors................................................................................ 68
6 Troubleshooting ........................................................................................................ 69
6.1 Patient positioning............................................................................................... 69
6.2 Image appearance .............................................................................................. 72
6.3 Artefacts .............................................................................................................. 73
6.4 Unit operation...................................................................................................... 75
7 Maintenance............................................................................................................... 77
7.1 Maintenance procedure ..................................................................................... 77
7.1.1 Annual maintenance ................................................................................ 77
7.1.2 Calibration intervals.................................................................................. 77
7.2 Changing the fuses ............................................................................................. 78
7.3 Cleaning and decontaminating the unit............................................................... 78
8 Calibration and adjustment ...................................................................................... 81
8.1 Introduction ......................................................................................................... 81
8.2 Preparing for calibration ...................................................................................... 82
8.3 Panoramic calibration.......................................................................................... 83
8.3.1 Panoramic geometry calibration............................................................... 83
8.3.2 Panoramic pixel calibration ...................................................................... 84
8.3.3 Panoramic Quality Check (optional)......................................................... 85
8.4 3D calibration ...................................................................................................... 87
8.4.1 3D geometry calibration ........................................................................... 87
8.4.2 3D pixel calibration................................................................................... 88
8.4.3 3D Quality Check program....................................................................... 89