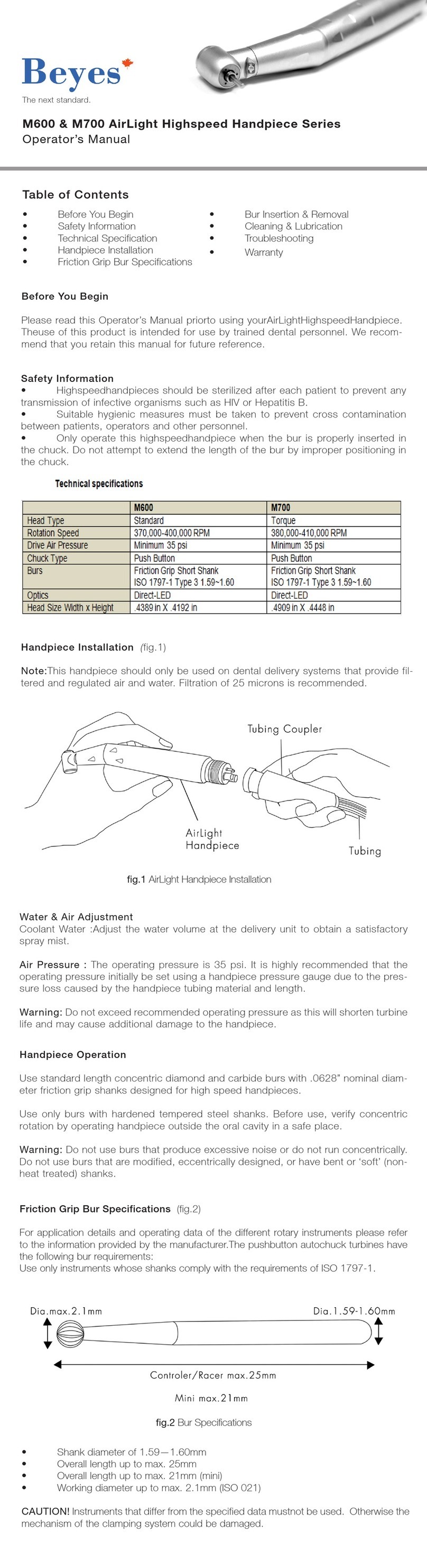
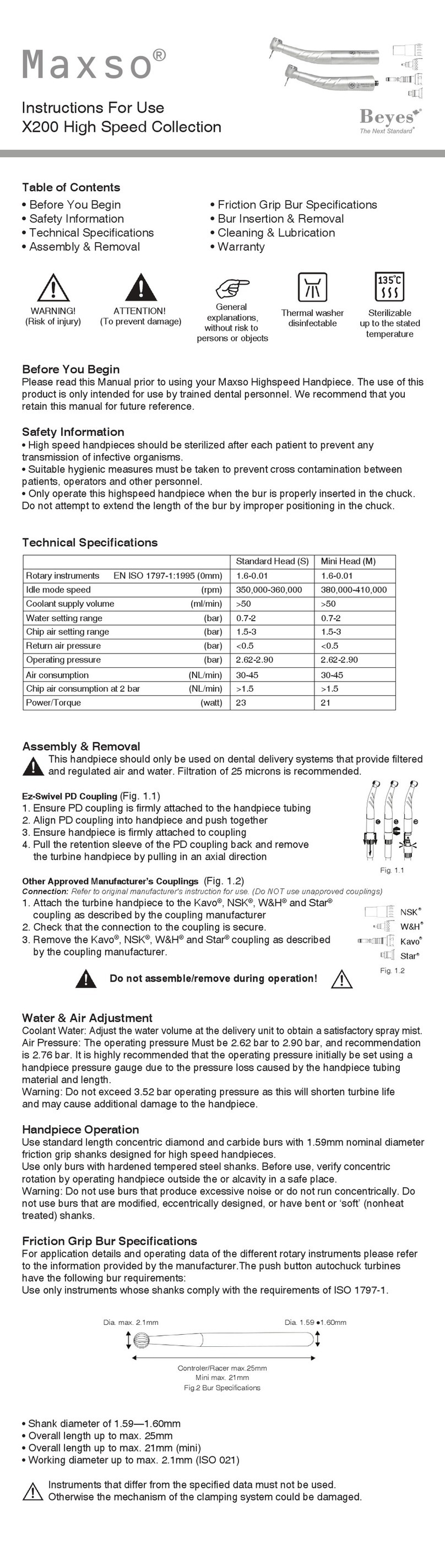
11
Fault Possible cause Soulutions
The scanling tip doesn’t
vibrate and there is no water
owing out when stepping
on the foot switch
The power pipe plug is in loosen
contact
Make the pulg insert to the socket
well
The foot switch is in loosen contact Insert the foot switch to its socket
tightly
The fuse in the main unit is broken Contact our dealers or us
The scaling tip doesn’t
vibrate The tip is in loosen contact Screw the tip on the handpice tightly
There is water owing out
when stepping on the foot
switch
(see gure7)
Something wrong with the handpiece Send the handpice to our company
to repair
Something wrong with the cable Contact our dealer or us
The scaling tip vibrates
but there is no spray when
stepping on the foot switch
The water control switch is not on Turn on the water control switch
[note 1]
There is impurity in the electric-
magnetic valve Contact our dealers or us
The watere system is blocked Clean the water pipe by multi-
function syringe[note 2]
There is still water owing
out after the power is off
There is impurity in the electric-
magnetic valve Contact our dealers or us
The handpiece generates
heat
The water control switch is in a low
setting
Turn the water control switch to a
higher grade[note2]
The amount of spouting
water is too little
The water pressure is not high enough Make the water pressure higher
The water pipe is blocked Clean the water pipe by multi-
function syringe[note2]
The vibration of tip becomes
weak
The tip hasn’t been screwed on to the
handpiece tightly
Screw the tip on the handpiece
tightly(see gure7)
The tip is loosen because of vibration Screw on the tip tightly(see gure7)
The coupling between the handpiece
and the cable isn’t dry Dry it by the hot air
The tip is damaged[note 3] Change to a new tip
There is water seeping from
the coupling between the
handpiece and the cable
The waterproof “O” ring is damaged Change to a new “O” ring
The ultrasonic doesn’t
vibrate
The screw is loosen Tighten it
Endochuck is damaged Change to a new endochunck
There is noise coming from
the endochuck The screw is loosen Tighten it
Peristaltic leak The inner water pipe cracks Replace a new peristaltic pump
There is no water
coming out from the
handpiece(automatic water
supply mode)
There is air in the water pipe Turn the water control to the Max,
reinsert the bottle.
3.5.1 Troubleshooting
3.5 Troubleshooting and notes