STERILUX SterOx System V Series User manual

SFT-0034-V03-IFU_SterOx_System_V-Series
Release date: 12 Nov. 2021
SterOx System V-Series
Ozone Sterilizer Instruction for Use

2

3
All rights reserved
No portion of this manual can be printed, transmitted, rewritten, stored in a data recovery
system, translated in any foreign or computer language, in any form or through any devices,
without written consent by SteriLux SA.
Information in this manual is subject to change without any warning or prior notice by
SteriLux SA.
SteriLux SA
Chemin du Viaduc 12
1008 Prilly – Switzerland
T: +41 (0) 21 546 43 33 | E: info@sterilux.ch | www.sterilux.ch

4
Table of contents
1 Introduction..........................................................................................................................................................................................................5
2 Symbols .................................................................................................................................................................................................................6
3 Important information..................................................................................................................................................................................... 7
3.1 Disclaimers .........................................................................................................................................................................................................................................7
3.2 Declaration of Conformity.............................................................................................................................................................................................................7
3.3 Field of application..........................................................................................................................................................................................................................7
3.4 Warning............................................................................................................................................................................................................................................... 8
4 Products overview..............................................................................................................................................................................................9
4.1 SteriBase V-Series (SKU-0006).................................................................................................................................................................................................... 9
4.2 SteriBox V-Series (SKU-0007)......................................................................................................................................................................................................10
5 Principles of operation....................................................................................................................................................................................11
6 Setting up your SteriBase V-Series............................................................................................................................................................ 12
6.1 Installing your device....................................................................................................................................................................................................................12
6.2 Powering your device....................................................................................................................................................................................................................13
6.3 Installing nitrogen supply............................................................................................................................................................................................................13
6.4 Setting the nitrogen flow..............................................................................................................................................................................................................14
6.5 Nitrogen purging.............................................................................................................................................................................................................................14
6.6 Connecting your device to a Wireless Network ...................................................................................................................................................................14
6.7 Setting the time and date............................................................................................................................................................................................................14
6.8 Setting the language.....................................................................................................................................................................................................................14
6.9 Setting the users/contents..........................................................................................................................................................................................................14
6.10 Managing email addresses.....................................................................................................................................................................................................................15
7 Using the SteriBox V-Series and preparing instruments ..................................................................................................................16
7.1 Using the SteriBox V-Series.........................................................................................................................................................................................................16
7.2 Preparing and loading instruments in the SteriBox V-Series .......................................................................................................................................... 17
8 Using your SterOx System V-Series .......................................................................................................................................................... 20
8.1 Preparing device for use.............................................................................................................................................................................................................20
8.2 Launching a cycle .........................................................................................................................................................................................................................20
8.3 Course of the cycle........................................................................................................................................................................................................................20
8.4 Interrupting a Cycle .......................................................................................................................................................................................................................21
9 Storing and retrieving cycle information............................................................................................................................................... 22
9.1 Retrieving cycle information from the history ..................................................................................................................................................................... 22
9.2 Retrieving cycle information using a USB stick................................................................................................................................................................... 22
10 Shutting device down...................................................................................................................................................................... 22
10.1 Normal shutdown.......................................................................................................................................................................................................................... 22
10.2 Emergency shutdown .................................................................................................................................................................................................................. 22
11 Maintaining your SterOx System V-Series............................................................................................................................... 23
11.1 Cleaning the SteriBox V-Series ................................................................................................................................................................................................. 23
11.2 Cleaning the SteriBase V-Series and exterior surface of the SteriBox V-Series ...................................................................................................... 23
11.3 Preventive maintenance............................................................................................................................................................................................................. 23
12 Troubleshooting your SterOx System V-Series ...................................................................................................................... 24
13 Spare parts .......................................................................................................................................................................................... 26
14 Recycling and disposal................................................................................................................................................................... 26
15 Warranty ................................................................................................................................................................................................27
16 Specifications..................................................................................................................................................................................... 28
17 Appendix .............................................................................................................................................................................................. 29
17.1 Electromagnetic compatibility – Environment ...................................................................................................................................................................29
17.2 Electromagnetic compatibility – Performance Levels .....................................................................................................................................................30
17.3 Accessories replacement............................................................................................................................................................................................................31

5
1 Introduction
Congratulations on selecting the SterOx System V-Series. The SterOx System V-Series is a
compact, environmentally-friendly sterilization device based on ozone as sterilizing agent
composed of two components: the SteriBase V-Series and the SteriBox V-Series.
The details of installing, operating, and maintaining your SterOx System V-Series are all
contained within these instructions for use manual. Please read these instructions before
starting to operate this device and keep them for future reference. Calibration tests were
performed at the factory; the sterilizer does not require any special commissioning settings.
Operational, maintenance and replacement instructions must be followed.
The SterOx System V-Series is suitable for terminal sterilization of metal and nonmetal
devices compatible with ozone sterilization. The SterOx System V-Series has not been
designed to sterilize liquids, textile fabrics, powders, bio-medical waste or any other
materials not compatible with ozone sterilization. The processing of such loads may result
in incomplete sterilization and / or damage to the load and / or to the sterilizer. For more
information about instruments compatibility with ozone sterilization, please contact
SteriLux SA directly.

6
2 Symbols
The following table displays the different symbols used in the margins of this manual and/or
on the product and summarizes their meaning.
Serial number
Catalogue number
Batch code
Date of manufacture
Manufacturer
Use-by date
CE marking
Consult instructions for use
Caution, consult the instructions for use for important cautionary information
Keep away from sunlight
Keep dry
Temperature limit
Humidity limitation
Fragile, handle with care
Waste Electrical and Electronic Equipment
Caution, hot surface
Caution, UV radiation
Direct Current
Alternative current
Stand-by / Switch button
Protective Conductor Terminal
Potential hazard to the operator

7
3 Important information
3.1 Disclaimers
The protection provided by the equipment may be impaired if the operator does not use the
SterOx System V-Series in a manner specified in the present document. Failure to follow the
present document instructions and recommendation may lead to machine malfunction
and serious injuries. Do not permit any person other than certified personnel to supply parts
for service or maintain your SterOx System V-Series. SteriLux SA shall not be liable for
incidental, special or consequential damages caused by any maintenance or services
performed on the SterOx System V-Series by a third party, or for the use of equipment or
parts manufactured by a third party, including lost profits, any commercial loss, economic
loss, or loss arising from personal injury.
Never attempt to remove the SteriBase V-Series cover by removing the screws. Doing so
may damage the device and/or pose a hazard to the operator.
The use of this sterilizer is limited to the range of application indicated in this technical
document and must only be operated with fully functional accessories, consumables and
spare parts recommended or supplied by SteriLux SA. SteriLux SA shall not be liable for
incidental, special or consequential damage caused by the use of products, accessories,
consumables or spare parts not recommended or supplied by SteriLux SA and/or damaged
or suspected of damage.
The operations of preparation and sterilization of devices must be carried out by qualified
personnel only.
It is imperative to sterilize only instruments and other devices that are specified as
sterilisable by the manufacturer.
3.2 Declaration of Conformity
The SterOx System V-Series falls into the definition of a Low Voltage Device, as defined by
Article 1 of 2014/35/EU.
The manufacturer declares under its sole responsibility, that the product listed below is in
conformity with:
1) Safety Objectives referred to in Article 3 and set out in Annex I of European Directive
2014/35/EU, as amended.
2) The relevant Essential Requirements of Article 3 of European Directive 2014/53/EU, as
amended, and that Annex III (Module B on EU-type Examination) has been followed for
their conformity assessment.
3) The relevant Essential Requirements of Article 4 of European Directive 2011/65/EU, as
amended.
3.3 Field of application
This sterilizer has been designed for indoor use only in veterinary settings.

8
3.4 Warning
Careful monitoring of the shelf-life and/or the maximum allowable number of cycles of the
different components of the SterOx System V-Series is essential to ensure the required
sterility assurance level (SAL) at the end of the cycle. Never use components that have an
expired shelf-life or exceeded the allowable number of cycles.
Do not remove or erase labels and markings present on individual products.
Always allow the sterilizer to cool down to room temperature before transporting and use a
suitable transport packaging.
Avoid pouring or splashing of water or liquids on the SteriBase V-Series as this may cause
short circuits. Do not place any liquid or liquid container of any type on the SteriBase V-
Series or close to it.
When using the SterOx System V-Series, ensure that the power cable port remains
accessible and removable at all time.
Keep the SterOx System V-Series away from children and pets at all time.
In case of malfunction or failure, the operator may be exposed to higher ozone
concentration. In case of suspected malfunction, shut the device down using the power
switch, ventilate and evacuate the room for at least 30 minutes.
Any person suspected to have come in contact with high ozone concentration or who has
effectively been in contact with high ozone concentration shall seek medical assistance
and follow-up as soon as possible.
The SteriBase V-Series contains lamps that emit ultraviolet (UV) radiations.

9
4 Products overview
4.1 SteriBase V-Series (SKU-0006)
1 Touchscreen
2 Start button
3 Handle
4 Manual levers (of the
Lamp Unit)
5 Cooling fan
6 Thermal printer
7 Nitrogen plug
8 Power switch
9 Power cable port
When you receive your SteriBase V-Series, the items listed below should be included. If any
of the items are missing or damaged, contact your supplier immediately.
• SteriBase V-Series
• Power cord
• Back door access key
• Instructions for use
• Short user guide
Note: The SteriBase V-Series has a 10-year shelf-life.
10
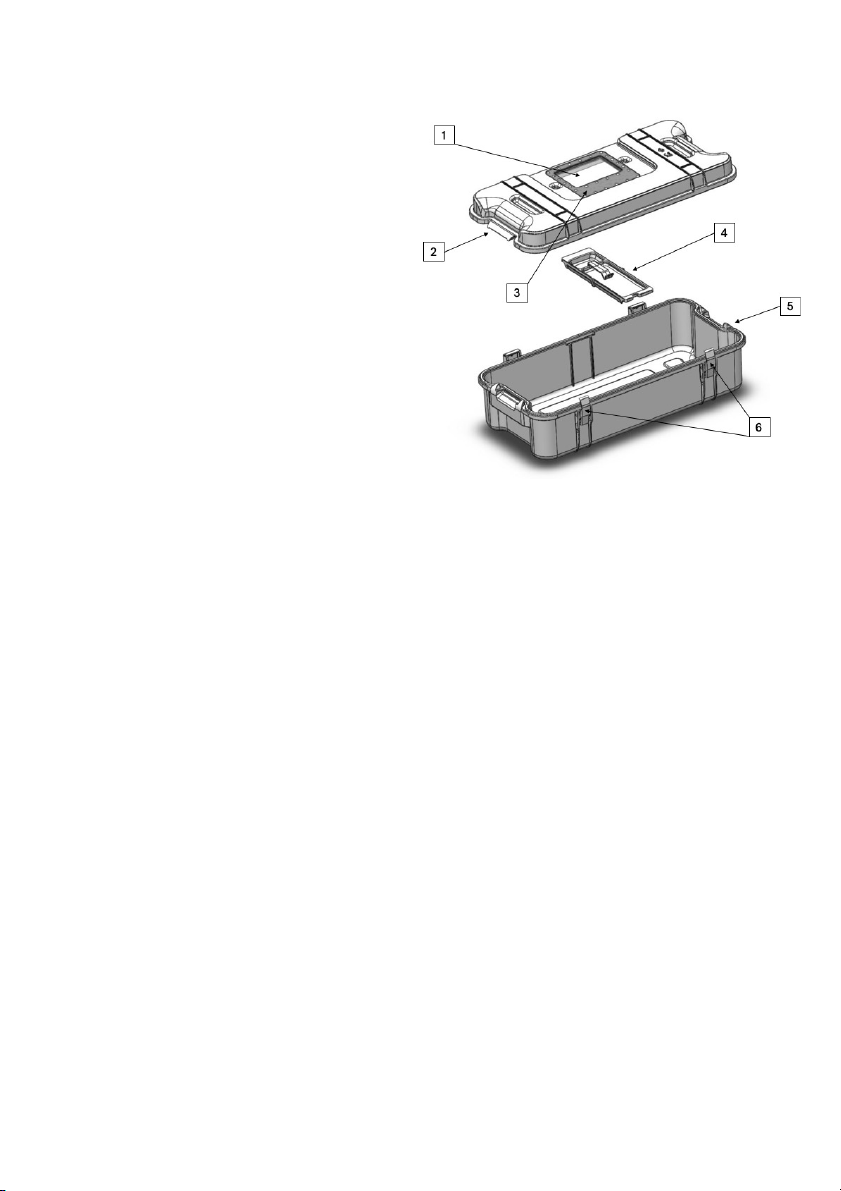
4.2 SteriBox V-Series (SKU-0007)
1 Quartz window
2 Lid
3 Incorporated RFID tag
4 Module and clip
5 Bottom
6 Locks
When you receive your SteriBox V-Series, the items listed below should be included. If any
of the items are missing, contact your supplier immediately.
• SteriBox V-Series Lid
• SteriBox V-Series Bottom
• SteriBox V-Series Module and clip
• Instructions for use
Always use parts belonging to the same serial number of a SteriBox V-Series assembly (Lid,
Bottom, Module and clip).
Note: The SteriBox V-Series has a 3-year shelf-life or 1’000 cycles.

11
5 Principles of operation
The SterOx System V-Series is an ozone-based sterilizer. 172 nm UV lamps contained in the
Lamp Unit inside the SteriBase V-Series [1] transform oxygen (O2) from ambient air into
ozone (O3) [2]. Ozone further reacts with water (H2O) to form hydroxyl and hydroperoxyl
radicals [3] which are responsible for the inactivation of micro-organisms causing infections
and disease transmissions [4]. At the end of the sterilization process, a 254 nm UV lamp also
contained in the Lamp Unit transforms remaining ozone back into oxygen [5].

12
6 Setting up your SteriBase V-Series
6.1 Installing your device
There are several factors that may affect the performance of your SteriBase V-Series. Please
review these factors and select a suitable location in which to install the device. After each
relocation of the sterilizer, installation must be carried out according to the protocol.
Safety of any system incorporating the device is the responsibility of the assembler of the
system.
When transporting the SteriBase V-Series, never carry the SteriBase V-Series alone. Two
persons shall carry it by using the specific handles present on the SteriBase V-Series. Always
keep the SteriBase V-Series vertical when out of its packaging.
The SterOx System V-Series is conceived for use outside patient surroundings. Neither the
SteriBase V-Series nor the SteriBox V-Series should be in direct contact with the patient.
• Temperature and Humidity
Avoid installing your SteriBase V-Series in direct sunlight or close to a heat or cold source
(e.g. vents or radiators). The operating temperatures have to be between 20 – 30°C with
relative humidity of 20 – 70%. Ideal room temperature is 23°C.
• Spacing
The cooling fans and vents of the SteriBase V-Series should remain uncovered and
unobstructed. Leave a minimum of 20 cm between the top, side and back of the device and
any wall or partition. It will ensure proper ventilation and facilitate removal of power supply
if necessary. The main power switch acts as a disconnecting device and must remain easily
accessible in case the device needs to be disconnected.
• Venting
The SteriBase V-Series should be operated indoor in a clean, dust-free environment, with a
good ventilation system.
• Work surface
The sterilizer must be placed on a flat and horizontal surface that can support a total weight
of 200 kg. Do not install the sterilizer near water sources.
• Altitude and pressure
The SteriBase V-Series and the SteriBox V-Series cannot be used in altitude superior to
2’000 m above sea level. The system must be used in an environment with atmospheric
pressure of 1atm ± 0,095. The SteriBox V-Series must not undergo pressure changes
exceeding 5’000 Pa.
• Electromagnetic environment
Your SteriBase V-Series has been tested and meets applicable standards for
electromagnetic emissions (class A product). In a domestic environment this product may
cause radio interference in which case the user may be required to take adequate
measures. For more information on electromagnetic compatibility, please refer to
chapter 17.
• Electrical requirements
The SterOx System V-Series has been designed to be powered from the power cable port.
Connect the sterilizer to an AC power supply, properly grounded and fused power sources

13
with the following voltage rating: single phase 110/240 V – 50/60 Hz – 3 A. The system must
be used within ± 10 % of the nominal voltage (110/240 V). Do not plug into multiple outlet
receptacles.
The SteriBase V-Series has been designed to withstand transient overvoltage up to levels of
Overvoltage category II.
The SterOx System V-Series must be connected to a mains socket outlet with a protective
earthing connection. The manufacturer will not be responsible for damages to the operator
caused by an unsuitable electrical installation or missing of the protective earth connection.
Always observe the electrical instructions, standards and regulations for the electrical
connection of the sterilizer. In case of doubt, please consult a qualified technician. It is
important to never modify, bend or twist the power cord. Never place heavy objects on the
cable or place it near a heat source. Do not use staples or nails to secure the cable. In case
of damage to the plug or cable, immediately disconnect the power supply. To completely
disconnect the power supply from the SteriBase V-Series, pull the separable power cord out
of the device and turn off power switch.
6.2 Powering your device
To power your SteriBase V-Series, connect the power cord to the power cable port (AC inlet
receptacle on the right side of the device). Ensure the power switch is in the OFF position
and connect the device to your power source. Turn the power switch in the ON position and
click on the Start button.
6.3 Installing nitrogen supply
To function properly, your SteriBase V-Series requires a nitrogen gas supply. SteriLux SA
proposes a turnkey solution that includes:
• 4 disposable nitrogen cylinders 110 bar 2.2L
• 1 reusable pressure regulator
• 1 m tubing external diameter 4 mm
To install it, remove the nitrogen cylinder protection from the bottle and firmly secure the
pressure regulator on the nitrogen bottle. Connect one end of the tubing to the
pressure reducer and open the nitrogen bottle until feeling a weak flow coming
out of the tube. Quickly connect the other end of the tube to the nitrogen plug
on your SteriBase V-Series. Check that the tubing is well connected by gently
pulling it at both ends. Keep opening the nitrogen cylinder until the needle on
the right dial reaches 4.5 bar (see photo). The left dial of the pressure regulator
indicates the amount of nitrogen remaining. When the bottle is empty (i.e.
needles on both dials are back to 0) change the bottle following the same
procedure.
Never use device without nitrogen supply or on an empty cylinder as this can permanently
damage the device and may impede the sterilization process.
Note: It is possible to use other commercially available nitrogen supply cylinders. However,
if you decide to go with another alternative, you must have SteriLux SA written approval
prior to installing it on your SteriBase V-Series.

14
6.4 Setting the nitrogen flow
1. Open the SteriBase V-Series back door using the access key provided with
the device
2. From the menu PARAMETERS click on Nitrogen flow setting- this will activate
nitrogen flow for 2 minutes
3. While the flow is activated, set it to 1.2 l/min (see photo)
Note: If the flow is not activated the ball will always be on 0.
6.5 Nitrogen purging
At device installation and if the device has not been used for more than 3 weeks, a nitrogen
purging should be performed. The goal of this program is to remove all traces of oxygen
from the Lamp Unit. Traces of oxygen in the Lamp Unit cause formation of ozone inside the
Lamp Unit thus preventing efficient irradiation of the UV lamps inside the SteriBox and
increasing the risk of cycle errors.
1. From the menu MAINTENANCE click on Nitrogen purging
2. This program lasts 17 minutes
6.6 Connecting your device to a Wireless Network
1. Go to the menu WIFI
2. Click on the three dots button and click on Connect to a Wi-Fi network
3. Select Wi-Fi, enter login details and press OK
Note: Connecting your device to a Wireless Network is essential for quick troubleshooting
from SteriLux and enables regular software updates.
6.7 Setting the time and date
1. From the menu PARAMETERS click on Date and time
2. Use the up and down buttons to set the date and time
3. Press the tick button to save your changes
Note: The date and time are automatically set if the device is connected to a Wireless
network
6.8 Setting the language
1. From the menu PARAMETERS click on Language
2. Select your desired language from the list – your selection will be automatically be
saved
6.9 Setting the users/contents
1. From the menu PARAMETERS click on User/Content settings
a. Create a new user/content by clicking on Add user/content
b. Edit existing users/contents by clicking on the user/content you want to
modify
c. Delete existing users/contents by clicking on the user you want to delete
2. When creating a new user/content or editing existing users/contents, a touchscreen
keypad will appear allowing you to create and modify your entries at any time - to save
your entries press Save, to discard press Cancel.

15
6.10 Managing email addresses
Provided the device is connected to a stable Wi-Fi network, you can inform one or more
email addresses to which the device will send a detailed PDF report at the end of each cycle.
1. In the menu PARAMETERS click on Email addresses
a. Enter new email address by clicking on Add email address
b. Edit existing email address by clicking on the email address you want to
modify
c. Delete existing email address by clicking on the email address you want to
delete
2. When adding a new email address or editing existing email addresses, a touchscreen
keypad will appear allowing you to create and modify your entries at any time - to save
your entries press Save, to discard press Cancel
3. Be careful not to add any blank space when typing the email address

16
7 Using the SteriBox V-Series and preparing instruments
7.1 Using the SteriBox V-Series
Opening the SteriBox V-Series in a sterile manner
1. Disengage both locks
2. Stand behind the SteriBox V-Series and grab the handles on both sides of the lid
3. Pull the lid towards yourself
4. Rest the lid on its outer surface
5. Take the module out with sterile gloves and place it inside the lid
Closing the SteriBox V-Series
1. Place module in the dedicated SteriBox V-Series notches
2. Align the lid with the bottom of the SteriBox V-Series
3. Engage the locks
4. Push the locks downward until they are secured
Inserting the SteriBox V-Series into the SteriBase V-Series
1. Slide the SteriBox V-Series inside the SteriBase V-Series with the locks facing you
2. Verify that the SteriBox V-Series is inserted all the way in
3. The touchscreen will automatically display the pop-up message SteriBox #
inserted
Removing the SteriBox V-Series from the SteriBase V-Series
1. If the Lamp Unit manual levers are in upward position, simply slide the SteriBox V-
Series out of the device
2. If the Lamp Unit manual levers are in downward position, grab both levers and pull
them up to upward position. Then slide the SteriBox V-Series out of the device
3. When removing the SteriBox V-Series from the SteriBase V-Series, exercise caution
as the quartz window area may be hot
Important information
The module is a highly important piece of the SteriBox. It serves not only to hold the blotting
paper with the 5 mL of water but also to measure the ozone concentration throughout the
cycle. The upper part of the module should always be clean and nothing should be placed
on top of it.
Never force the lid to close as this might alter the physical integrity of the SteriBox V-Series.
Always handle the lid with care as the quartz window is very fragile, it has been tested to
withstand 1 Joule of Impact, corresponding to a normalized impact rating IK06.
The SteriBox V-Series shall be stored in a clean and dry environment. Do not stack more
than 3 SteriBox V-Series on top of each other. The SteriBox must be stored on a flat surface
that can support up to 20 kg.

17
7.2 Preparing and loading instruments in the SteriBox V-Series
Before loading any instruments in the SteriBox V-Series, consult the instructions for use or
SteriLux SA directly to check for instruments compatibility. The maximum load to be
sterilised – including the weight of any sterilization basket, rack or other accessories – must
not exceed 7.5 kg, otherwise SteriLux SA cannot guarantee sterility of the load.
SteriLux SA guarantees the performance of its products only when their physical integrity
has not been altered. In the event of leaks, cracks or other damage, or suspected damage,
the equipment should not be used. Therefore, an inspection must be carried out before each
use. In case the quartz window presents a crack, do not use as the airtightness of the
SteriBox V-Series may be altered and the operator risks being exposed to higher ozone
concentration.
Clean Instruments
Clean, rinse and dry all instruments before loading them into the SteriBox V-Series.
Disinfectant residues and solid debris may inhibit sterilization and damage the instruments.
Lubricated instruments must be wiped thoroughly, and any excess lubricant should be
removed before loading. Improper cleaning, rinsing or drying can cause the sterilization
cycle to malfunction. Breach of the appropriate preparation instructions can lead to non-
sterility of the instruments. Sterility cannot be guaranteed if the material to be sterilized is
dirty, contains dust, residues or biofilms.
Avoid glutaraldehyde- and peracetic acid-based disinfectants. Here is a list of preferred
commercially available disinfectants to be to disinfect medical devices to be sterilized in
the SterOx System V-Series:
1. STABIMED® FRESH – B BRAUN
2. GIGASEPT® INSTRU AF – SCHÜLKE
3. NEODISHER® SEPTO PRECLEAN – DR. WEIGERT
4. BOMIX® PLUS – HARTMANN
Add distilled water on the HUMIDIFY Blotting Paper
For effective sterilization, it is mandatory to add 5mL of distilled water on the HUMIDIFY
Blotting Paper provided by SteriLux. If there is any doubt about the amount of water added,
remove the HUMIDIFY Blotting Paper and dispose of it. Dry the module and repeat the
operation with a new HUMIDIFY Blotting Paper. A smaller or larger amount of water will not
guarantee effective sterilization. Refer to the HUMIDIFY Blotting Paper Instructions for Use
for detailed instructions.
Unwrapped instruments
You can arrange unwrapped instruments either directly in the SteriBox V-Series as it is, or in
a sterilization basket (the SteriBox V-Series has been designed to fit with standard size
sterilization baskets). Avoid stacking or pilling of instruments in the SteriBox V-Series, as this
might impede the sterilization process.
Wrapped instruments
Place the instruments into single sterilization pouches. Tyvek® sterilization pouches have
been cleared for use and are recommended for use with the SterOx System V-Series. Place
the wrapped instruments in the SteriBox V-Series or on a rack and arrange them to avoid
overlap. Always ensure that porous side of the pouches are facing upwards. Avoid
compressing of the pouches as this might impede the sterilization process.

18
Compatibility of materials
1
The following materials are compatible with the ozone sterilization process (non-exhaustive
list).
• Stainless Steel
• Glass
• ABS
• Acrylic (PMMA, PlexiglasTM)
• Polycarbonate (LexanTM)
• Polypropylene
• PTFE (TeflonTM)
• Polysulfone (UdelTM)
• Polyetherimide (UltemTM)
• PEEK
• Polyethylene
• Polyoxymethylene
• PVC
• Silicone
The following materials ARE NOT compatible with the ozone sterilization process (non-
exhaustive list):
• Natural rubber
• Nitrile
• Nylon
• Latex
Use of these materials may lead to instrument or equipment damage. If you are unsure of
your instrument’s material or construction, do not load into your SterOx System V-Series
until you have checked with the instrument manufacturer and/or SteriLux SA.
All instruments
The SterOx System V-Series has not been designed to sterilize liquids, textile fabrics,
powders, bio-medical waste or any other materials not compatible with ozone sterilization.
Instruments will remain sterile after a successful cycle until the locks of the SteriBox V-Series
are disengaged (maximum storage of sterile instruments 1 year). Unwrapped instruments,
once exposed to ambient or external conditions, cannot be maintained in a sterile state. If
sterile storage is desired, either leave the SteriBox V-Series securely closed, or wrap the
instruments to be sterilized in sterilization pouches.
It is proscribed to reprocess tools that came in contact with a patient suffering or suspected
to suffer from any form of prion-related diseases (e. g. Creutzfeldt-Jacob disease (CJD), the
new variant of CJD, Gerstmann-Sträussler-Scheinker syndrome, Kuru's disease, Fatal
Familial Insomnia, Scrapie, Feline spongiform encephalopathy). Complete destruction of all
prions cannot be guaranteed.
Routine Monitoring
SteriLux SA provides two types of chemical indicators to monitor the sterilization process:
• Ozone Strip type 1 Chemical indicator – to be used in all cycles (Speed, Standard and
Pouch) as an external or internal pack process indicator to exposure to ozone in the
SterOx System V- Series
1
Only stainless steel has been cleared for use in the SterOx System V-Series, other materials show good
compatibility with ozone in the literature.

19
• Ozone Strip type 4 Chemical indicator – to be used exclusively in Standard and Pouch
cycles as a multi-variable indicator responding to all variable parameters
For more information regarding both chemical indicators refer to their related instructions
for use.
Note for hollow instruments
SteriLux SA cannot guarantee that the inside of hollow instruments can be sterilized by the
SterOx System V-Series. Overall, channels with greater length than 10 cm and smaller inner
diameter than 3 mm of material different than stainless steel have not been cleared to be
reprocessed in the SterOx System V-Series.

20
8 Using your SterOx System V-Series
8.1 Preparing device for use
Once the device is installed and before any cycle is launched, clean the lid, bottom and
module of the SteriBox V-Series (see chapter 11 for further details). Pay special attention to
thoroughly clean the quartz windows, both on the SteriBox V-Series and the SteriBase V-
Series, using any commercially-available glass cleaner and a clean soft cloth.
8.2 Launching a cycle
The SterOx System V-Series proposes three cycles.
The Speed cycle is designed for maximum flexibility. This cycle was validated on all
common pathogens encountered in the veterinary setting for wrapped and/or unwrapped
simple geometry devices only. The cycle takes place at room temperature and ambient
pressure and lasts 3 hours.
The Standard cycle is designed for maximum security to sterilize all unwrapped instruments
compatible with ozone sterilization. This cycle was validated on Geobacillus
stearothermophilus spores. Unwrapped instruments will remain sterile after a successful
cycle as long as the SteriBox V-Series is kept closed (up to 1 year). Once exposed to ambient
or external conditions, unwrapped instruments cannot be maintained in a sterile state. The
cycle takes place at room temperature and ambient pressure and lasts 16 hours.
The Pouch cycle is designed for maximum security to sterilize all wrapped instruments
compatible with ozone sterilization. This cycle was validated on Geobacillus
stearothermophilus spores. The sterile storage time for wrapped instruments outside the
SteriBox V-Series is the time specified by the manufacturer of the sterilization pouches used.
The cycle takes place at room temperature and ambient pressure and lasts 22 hours.
To launch any of these three cycles:
1. Insert SteriBox V-Series inside the SteriBase V-Series
2. From the HOME menu press START NEW CYCLE
3. Select the cycle of your choice by pressing Select cycle
4. Optional: Select the user and content
5. Click on the start icon button
After clicking on the start icon button, the Lamp Unit will automatically drop down securing
the SteriBox V-Series inside the SteriBase V-Series.
During the first 15 minutes of each cycle, sounds of air under pressure being released at
regular intervals should be heard. If this is not the case, check that nitrogen supply is well
connected and not empty.
8.3 Course of the cycle
Throughout the whole cycle, the cycle phase, time remaining and general information
related to the cycle are displayed on the screen.
Other manuals for SterOx System V Series
2
Table of contents
Other STERILUX Laboratory Equipment manuals
Popular Laboratory Equipment manuals by other brands

Cole Parmer
Cole Parmer HP-200 Series instruction manual

METER
METER AQUALAB 3 manual

Thermo Scientific
Thermo Scientific Finnpipette F2 Instructions for use

10x Genomics
10x Genomics Chromium X Series Quick reference card

Agilent Technologies
Agilent Technologies 708-DS Training manual

Drummond
Drummond Pipet-Aid XL 4-000-105 Operation manual












