SurgEase LumenEye X1 User manual
LumenEye®X1
Instructions for Use

IFU-101-1.0
Contents
CE Markings
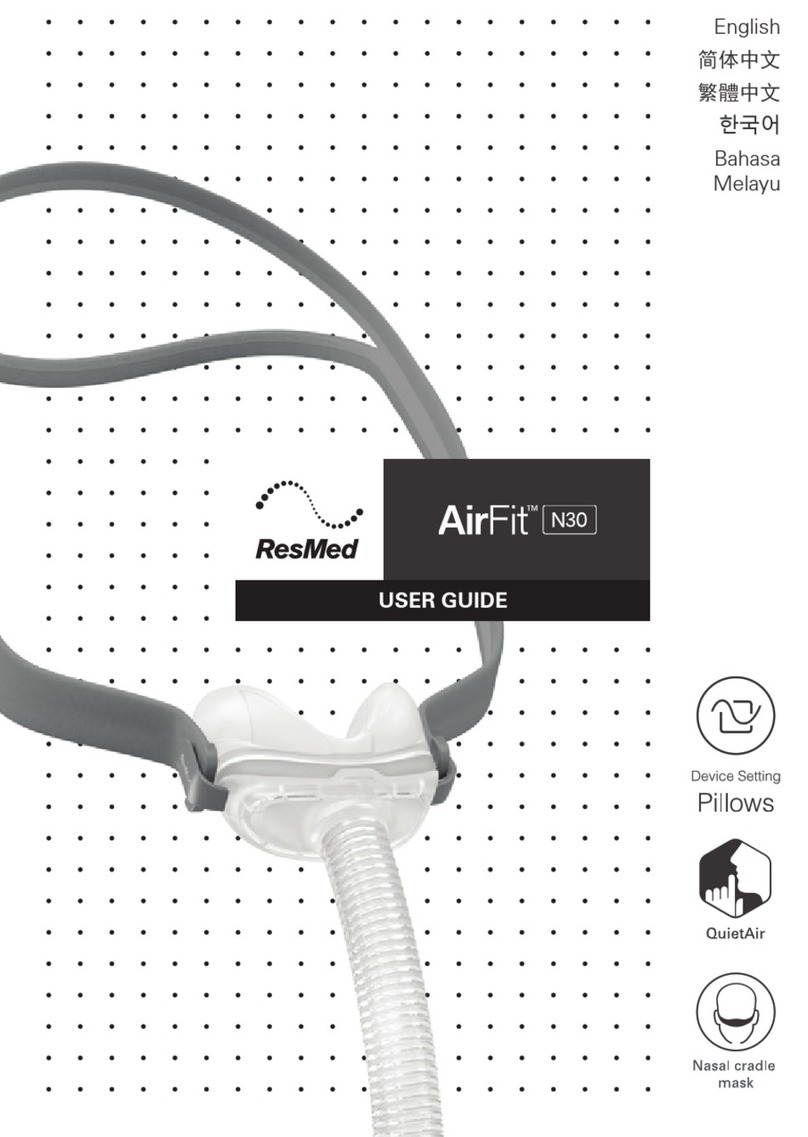
LumenEye®X1 system
LumenEye® X1 Medical Device
Intended Use
Contraindications & Warnings
Caution
Principle of Operation
Symbols
Docking Case - Getting Started
Docking Case - Cleaning, Disinfection and Maintenance
LumenEye®X1 - Assembly & Use
LumenEye®X1 - Cleaning & Disinfection: Preparation
LumenEye®X1 - Cleaning & Disinfection: Instructions
Charging Instructions
Software Compatibility
Warranty
Life of Product & Servicing
Disposal
Troubleshooting
Environmental Information
1
2
3
4
5
6
6
7
8
9
10
12
13
15
16
16
16
17
18
19
IFU-101-1.0
Copyright 2020 SurgEase Innovations Ltd referred to in this document as
‘SurgEase’. All rights are reserved. No one is permitted to reproduce or
duplicate, in any form, this manual or any part thereof without the explicit
permission of SurgEase. SurgEase assumes no responsibility for any illegal or
improper use of the product, that may result from failure to use this product in
accordance with the instructions, cautions, warnings, or statement of intended
use published in this manual. This document forms the Instructions for Use and
technical description of the LumenEye®X1 system.
Trademarks
LumenEye®is a European registered trademark of SurgEase (registration number
017947970).
Manual Overview
This manual applies to the LumenEye®X1 system; catalogue ref: LX1-PCK-201
(USB-C), LX1-PCK-202 (USB-A)
The LumenEye®X1 system which includes thefollowing components:
• LumenEye®X1 endoscope medical device, catalogue ref: LX1-SCP-201
(USB-C), LX1-SCP-202 (USB-A)
• Portable docking case & tablet; catalogue ref: LX1-DCK-201 (USB-C),
LX1-DCK-202 (USB-A)
• Lumened manifold with sheath & obturator consumable set; catalogue ref:
LX1-CSB-201
• Lumened manifold with sheath, obturator and camera cover consumable
set; catalogue ref: LX1-CCS-201
Please contact SurgEase directly to report any incidents and concerns
regarding safety, performance, and for general feedback and enquiries.
Please direct all complaints to the manufacturer or authorised distributor. In the
event of a serious incident resulting from the use of the device, the relevant
competent authority should be notified immediately.
The CE mark on these products indicate conformance with the
provisions of Medical Device Regulation 2017/745.
Manufacturer
SurgEase Innovations Ltd,
Suite 201, Pendle Business Centre
Commercial Road
Nelson, Lancashire
BB9 9BT
United Kingdom
+44 (0)330 043 6989
Authorised Distributor
1. CE Markings
1

IFU-101-1.0
2. LumenEye®X1 system
1
4
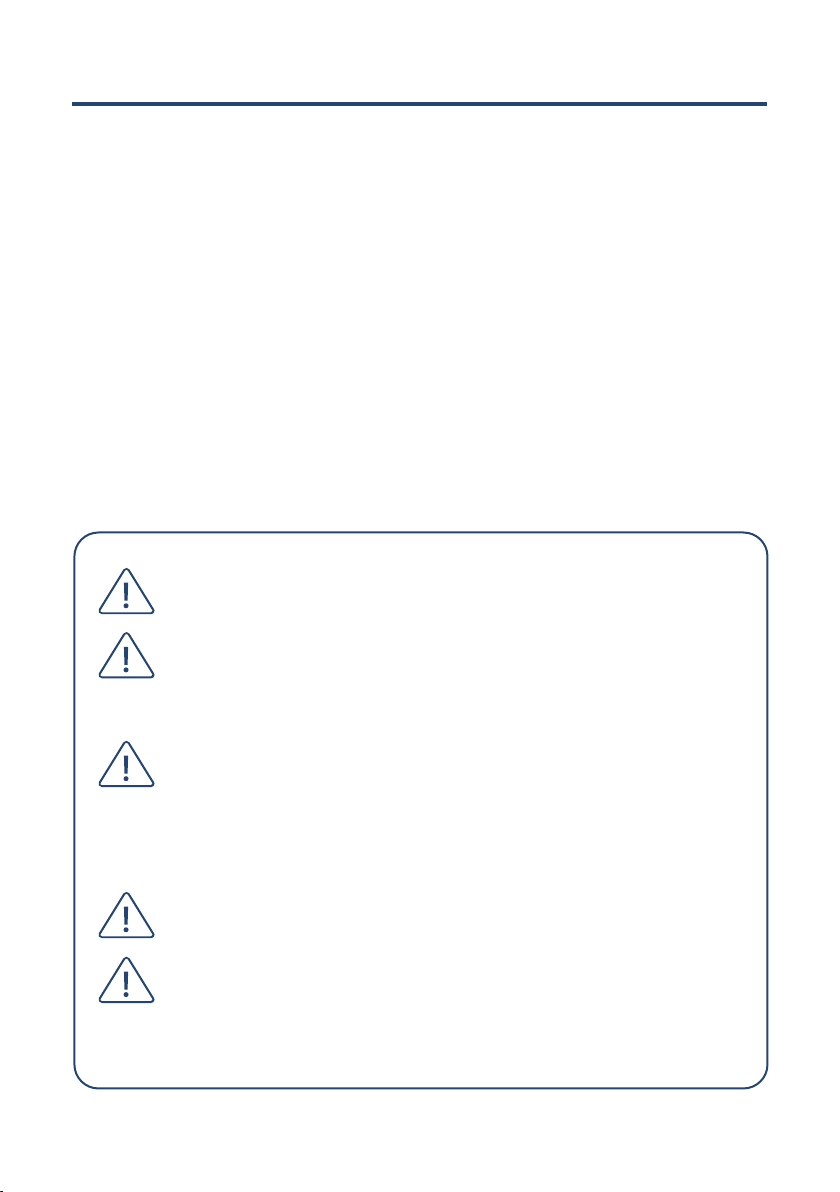
LumenEye®X1 system Components
1 LumenEye® X1 portable docking case
2 Storage for LumenEye® X1 consumables and tablet charger
3 LumenEye® X1 storage recess
4 LumenEye®X1 cable-tidy recess
5 LumenEye®X1 device mount
6 LumenEye®X1 handheld endoscope
7 Integrated keyboard
8 Touch screen tablet
9 Tablet case
2
5
7
8
3
6
9
2

IFU-101-1.0
3. LumenEye®X1 Medical Device
LumenEye® X1 Medical Device Parts List
1 LumenEye®X1 handheld endoscope
1.1 Camera and LEDs
1.2 Stainless steel camera tube
1.3 Air barb
1.4 Compliance label
1.5 Insuation bellows
1.6 USB connector
2 Sheath (single-use, applied part)
3 Obturator (single-use)
4 Manifold (single-use)
5 Camera Cover (single-use, optional part)
1
1.5
1.1
1.4
2
3
4
1.6
32
5
1.2
1.3
IFU-101-1.0
Caution: Users of the LumenEye®X1 should have suitable training
on rigid sigmoidoscopy techniques.
Caution: Users must read these instructions in full and ensure they
have a reasonable understanding of the procedure prior to using
the device. Questions should be directed to the manufacturer or
the local account manager.
Caution: The LumenEye®X1 device requires a single patient use
manifold, sheath and obturator. These components must not be
re-used and be discarded in clinical waste once a procedure is
completed. Components must only be purchased from SurgEase
or an approved distributor. Do not attempt to use the LumenEye®
X1 with other single-use components.
Caution: Do not attempt to insert a sheath without the obturator.
Attempting to do so may cause injury.
Caution: Each hospital or practice should maintain a robust
system for individual LumenEye®X1 traceability and ensure
that cleaning and disinfection audits are conducted regularly
(see section 12 & 13 for detailed cleaning and disinfection
instructions).
4. Intended Use
The intended use of the LumenEye®X1 system is:
• Examination of patients experiencing symptoms of peri-anal discomfort
and rectal bleeding.
• Detection of anal disease, rectal polyps, haemorrhoids, cancerous growths
and related bowel conditions aecting the rectum and anus.
• Surveillance of anorectal disease.
The device permits views of the anorectum and supports a clinician’s
examination so a diagnosis can be reached. Following an examination, further
investigations may be performed. The LumenEye®X1 manifold is a single-
patient use component which houses a channel to permit mucosal biopsy of
growths and lesions when clinically indicated. The device does not provide a
diagnosis and should not therefore be considered a diagnostic tool.
A typical rectoscopy procedure lasts up to 5 minutes. Total patient interaction
time is not likely to exceed 30 minutes. This may vary according to clinical need.
4

IFU-101-1.0
5. Contraindications & Warnings
Using the LumenEye®X1 in patients with BSE/TSE, HIV, Hepatitis C or other
communicable pathogens transmittable through direct physical contact
should be undertaken in consideration of local standard operating procedures
on infection control. Please see section 12 & 13 for further details on cleaning
and disinfection.
Users of the LumenEye®X1 must be suitably trained on performing rigid
sigmoidoscopy and proctoscopy. Users must read these instructions and ensure
they have an adequate understanding of the procedure prior to using the
device. Any questions should be directed to the manufacturer or the local
representative.
Warnings:
• Do not attempt to use the LumenEye®X1 with other commercially available
proctological single-use devices.
• Please inspect the LumenEye®X1 between uses. Particular attention should
be given to the camera lens to ensure there are no cracks or signs of
physical damage.
• Do not use biopsy forceps larger than 3mm.
• Only insuate air with the bellows until adequate views are achieved with
consideration of patient comfort.
• If the LumenEye®X1 or portable docking case and tablet become soiled,
they should be cleaned according to the instructions detailed in sections
10,12 and 13 of this document or notify the manufacturer if it cannot
be suitably cleaned. Do not use if there is uncertainty of the cleaning
robustness.
• If water enters the integrated bellows in the handle, do not use, and notify
the manufacturer.
• Do not attempt to dismantle or repair the device. This will void the warranty.
• Do not use defibrillation equipment whilst the device is in contact with the
patient. This may damage the camera and the electrical circuits.
• Do not attempt to modify the equipment.
• Avoid use in oxygen-rich environments and do not use in the presence of
flammable agents.
• If the equipment is used adjacent to, or stored alongside other electronic
equipment, the device should be observed carefully to verify normal
operation.
• Do not attempt to open the LumenEye®X1 scope or repair the electronics.
Opening the device may cause damage and will void the warranty.
• There are no user serviceable parts in the handheld unit or the
components.
• The user should check to ensure the view observed through the device
provides a live image (rather than a stored one) and has the correct image
orientation.
4 5

IFU-101-1.0
6. Caution
7. Principle of Operation
The LumenEye®X1 is comprised of an integrated CMOS camera and LED ring
which provide views and illumination of the anorectum. The integrated bellows
insuates the rectum to provide unobstructed views. The manifold is a sliding
attachment which has a biopsy channel allowing endoscopic biopsy and
retrieval of tissue. Existing 3mm endoscopic biopsy forceps should be used for
this function. Larger diameter forceps will not fit the channel and should not be
used.
6
The LumenEye®X1, docking case and tablet should never be stored or
operated in areas where they can become wet or be exposed to extreme
environmental conditions like high temperature, humidity, direct sunlight and
dust.
Do not autoclave, sterilise, or immerse this device in liquid or use caustic or
abrasive cleaning and disinfection agents. Please contact the manufacturer to
discuss suitable sterilisation options.
If this device fails to perform as intended, please contact the manufacturer. Do
not attempt to service this device.
This equipment complies with IEC 60601-1-2:2001 for Electromagnetic
Compatibility (EMC) concerning medical electrical equipment and/or
systems. This standard is designed to provide reasonable protection against
harmful interference in a typical medical installation. However, because of the
growing number of radiofrequency transmitting equipment and the general
electrical noise in healthcare environments, it is possible that high levels of such
interference from close proximity or strength of transmissions could disrupt the
performance of the device. Medical electrical equipment requires special
precautions concerning EMC, and all equipment must be installed and placed
into service according to the EMC information specified in this Instructions for
Use. The device should not be connected to any other medical device. If a
user opts to do so, then the obligation is on them to ensure the connected
device also complies with IEC 60601-1-2:2001. EMC results are available upon
request.
In compliance with the European Directive on Waste Electrical and Electronic
Equipment (WEEE) 2002/96/EC, do not dispose of this product as unsorted
municipal waste. This device contains WEEE materials; please contact SurgEase
regarding return or recycling of the LumenEye®X1.
Do not disassemble, heat above 100°C (212°F) or incinerate.
If any serious incident occurs in relation to the device, the user should report the
incident to the manufacturer and relevant competent authority.

IFU-101-1.0
8. Symbols
Indicates name and address of the legal manufacturer
Prefixes date of manufacture
The product is a medical device as defined by Medical Device
Regulation 2017/745
Serial number
Lot number
REF description should state “catalogue reference”
Refer to instruction manual
Note: This symbol is blue on the product label
Direct current power
Voltage: 5V DC
Current: 500mA
Cautions and Warnings
Equipment providing a particular degree of protection (isolation)
against electric shock particularly regarding allowable leakage
currents having a BF type (floating) applied part
Particulate and water ingress
Device handle: IP 23
Camera Housing: IP 67
Keep the system dry when packaged for storage and transportation
Please store between 10°C and 40°C
1 2
IPN N
76
REF
The intended users of the LumenEye®X1 should be trained healthcare
professionals. Users of the system are expected to have a pre-requisite level
of medical knowledge and experience in order to review and analyse the
captured data.
LOT
Note: All labels are printed in black
10°C
40°C
IFU-101-1.0
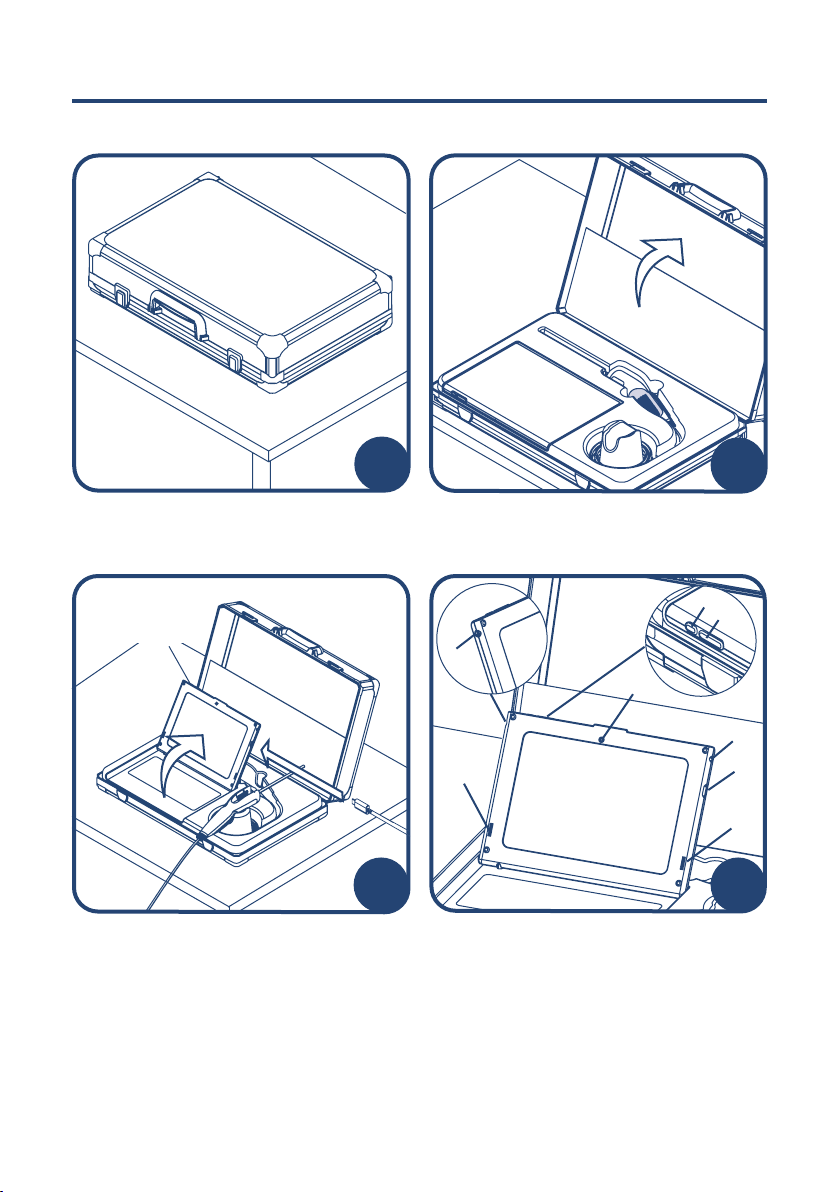
Open the docking case lid to a fully
retracted position and remove all
packaging.
Open the tablet screen to preferred angle
(1), connect the LumenEye®X1 to the USB
port (2), press the ‘power on’ button (3)
and refer to ‘IFU-401’ for CHiP software
instructions. (Please note: the tablet is
controlled via a touch screen interface.
Please use the integrated keyboard for
typing).
Tablet Feature List
1 Integrated front-facing webcam
2 Power adapter port
3 LumenEye®X1 USB port
4 Speakers
5 Headphone port
6 Power on/o
7 Volume control (-/+)
2
3
4
9. Docking Case - Getting Started
Setting Up
Ensure the docking case is placed on
a solid, flat and clean surface before
opening.
3
1
1
2
3
4
4
67
5
2
34
12
3
8
POWER
BUTTON HERE
IFU-101-1.0
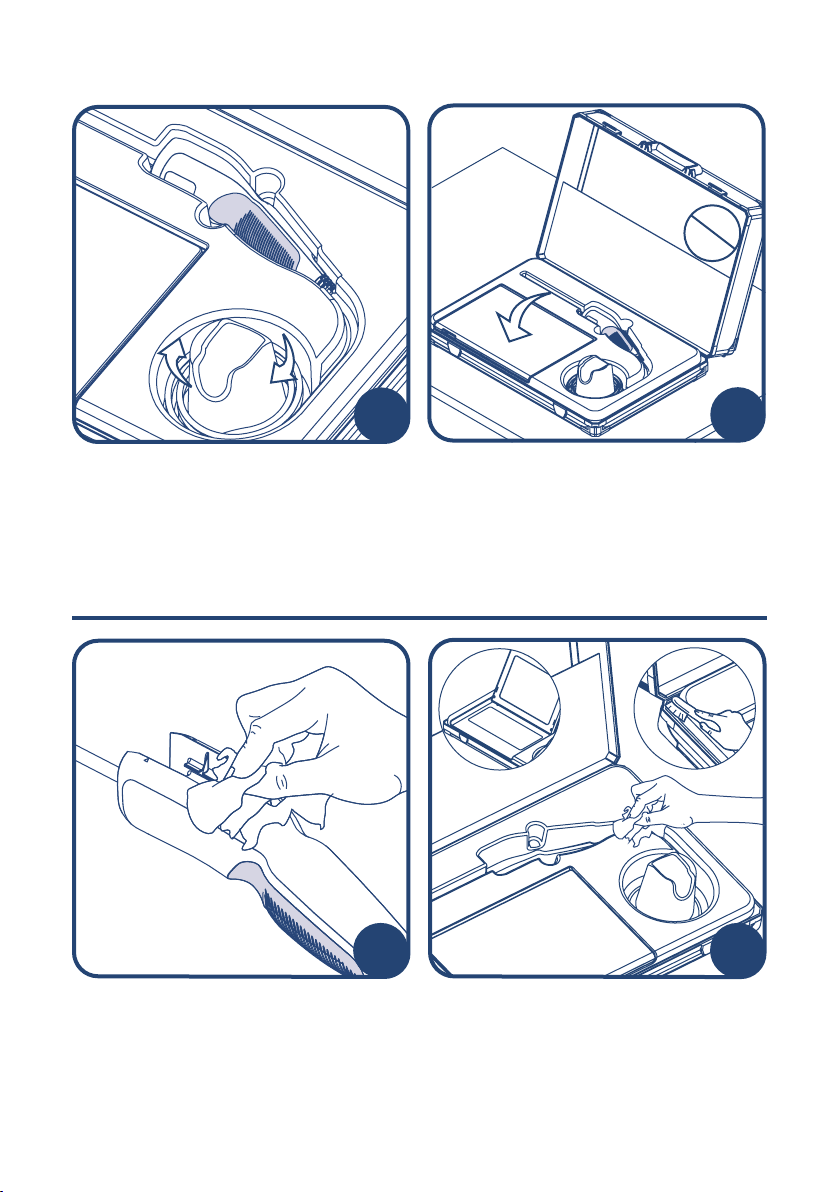
Ensure that the tablet is fully switched o
and the tablet screen is placed in the
closed position before shutting the case lid.
Ensure there is no obstruction of the mount
when closing the case lid.
To prevent unnecessary damage, ensure
the LumenEye®X1 is packed in the
dedicated storage recess and the cable is
wrapped in the cable-tidy recess as shown.
12
Storage
Before and after using the docking case
please ensure that all internal surfaces
have been cleaned with isopropyl wipes
including the tablet screen (1), keyboard
(1) and corners (2). A soft-bristled brush
may be used to manually clean any
crevices.
10. Docking Case - Cleaning, Disinfection and Maintenance
Prior to using and storing the LumenEye®
X1 please ensure the device has been
cleaned in accordance with the cleaning
and disinfection instructions in section 12
& 13.
5
1 2
6
12
98
DO NOT
OBSTRUCT
IFU-101-1.0
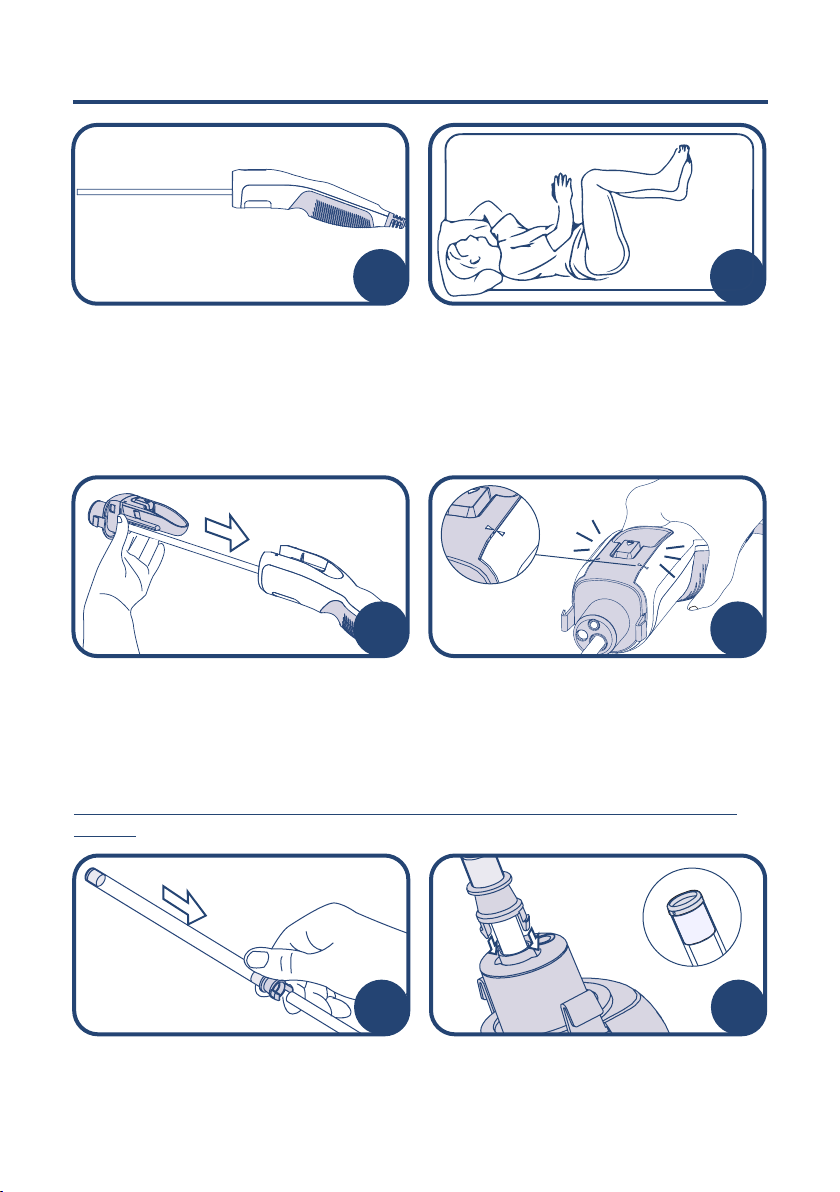
11. LumenEye®X1 - Assembly & Use
The patient should preferably be examined
in the left lateral position.
Perform a visual inspection to ensure the
device is clean. Check for visible cracks or
physical damage. Connect the camera to
the tablet, check all LEDs are illuminating
suciently and confirm the camera feed is
working.
Remove the disposable manifold from the
packaging and slide it over the LumenEye®
X1 camera tube via the opening marked
with a camera symbol.
Ensure the manifold is fully in place. An
audible click will be heard and the arrows
on the manifold will align with those on
the LumenEye®X1 handset indicating full
engagement.
12
3 4
CLICK!
TOP VIEW
10
If your consumable pack does not contain a camera cover please move to
step 7.
Push the camera cover into the slots firmly
until an audible snap is heard. Ensure
secure fitting by testing integrity with a
gentle pull.
Remove the camera cover from the
packaging and slide the connector over
the LumenEye®X1 camera tube.
56

IFU-101-1.0
Upon completion of the examination,
push the release button on the manifold
to disengage the LumenEye®X1 from the
sheath.
10
Remove the LumenEye®X1 from the
sheath and manifold, leaving the sheath
and manifold inside the patient. Air will
escape through the camera opening as
soon as the LumenEye®X1 is removed.
Allow sucient time for all insuated air to
escape.
11
1110
12
Carefully remove the sheath and manifold
from the patient and discard in clinical
waste.
INSIDE PATIENT
Insuate the appropriate amount of air by
squeezing the bellows firmly.
Once inserted, remove the obturator
whilst leaving the sheath inside the patient
and insert the LumenEye®X1 through the
sheath. Engage the manifold clips onto the
sheath until an audible click is heard.
Push the obturator fully into the sheath then
carefully insert into the patient. Please note
that the arrow on the sheath and obturator
should be facing 12 o’clock when insertion
is attempted.
78
1
2CLICK!
INSIDE PATIENT
INSIDE PATIENT
9

IFU-101-1.0
12. LumenEye®X1 - Cleaning & Disinfection: Preparation
Caution: After use, cleaning and
high-level disinfection of the
LumenEye®X1 must be carried
out after each use.
Caution: Before use, the
LumenEye®X1 must be visually
inspected & cleaned with an
isopropyl wipe to ensure the
device is clean and ready to use.
What you will need:
1) Tristel Trio Wipes system comprising of:
• Tristel Pre-Clean Wipes for the cleaning step. Tristel Pre-Clean Wipes are impregnated
with a low-foaming surfactant and a triple enzymatic detergent.
• Tristel Sporicidal Wipes and Activator Foam for high-level disinfection. Tristel Sporicidal
Wipe is activated with Tristel Activator Foam. Activated Sporicidal Wipe is a high-level
disinfecting wipe utilising Tristel’s chlorine dioxide chemistry.
• Tristel Rinse Wipes for the rinsing step. Tristel Rinse Wipes are sterile and impregnated
with deionized water.
2) Moistened Gauze Pad x 2
3) Soft bristled brush
4) Wet lint free cloth
5) Q Tip x 3
Cleaning Notes:
Do not re-use cloths or wipes. Soap, detergents or enzymatic cleaners should be used in
accordance with the manufacturer’s instructions. SurgEase is not responsible for damage
incurred during the cleaning process with products where no material compatibility
evaluation has been performed.
Where heavy faecal soiling has occurred and water irrigation is required, be careful
not to expose the handle to moisture or liquids. If the functionality of the device has
been compromised or there are water ingresses in the handle, please contact the
manufacturer.
Caution: Ensure the LumenEye®X1
is disconnected during ANY
cleaning and disinfection cycle.
12
Caution: Do not attempt
to reprocess this device by
autoclave.
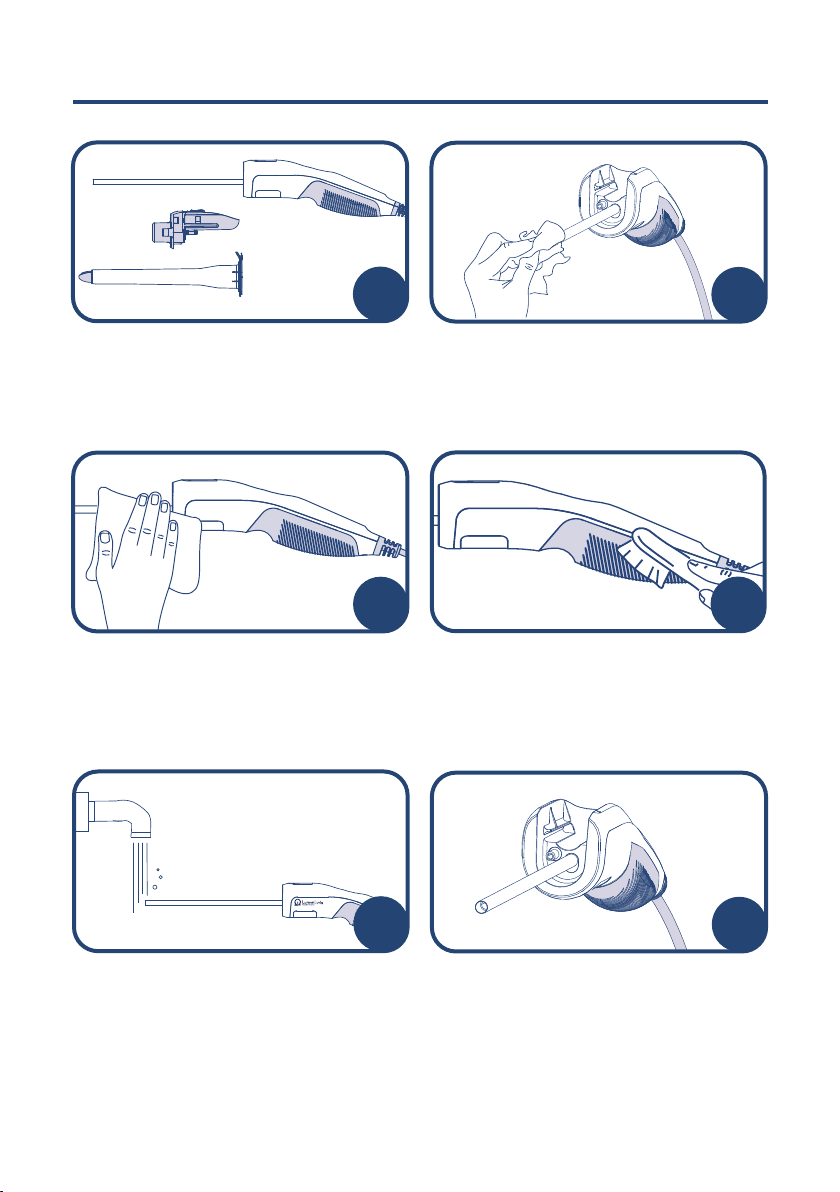
IFU-101-1.0
After each examination, ensure all lubricant
gel is wiped completely from the camera
tip. The device should not be left soaking
in gel.
After use, remove and discard the sheath
and manifold from the LumenEye®X1.
Single use parts should be disposed as
clinical waste.
A soft-bristled brush may be used to
manually clean any crevices, parting lines,
or irregular surfaces.
Remove visible debris as much as possible
using a moistened gauze pad. Exchange
the gauze pad if it becomes soiled.
If necessary, run only the endoscope tip
under running water and wash the handle
with a wet lint-free cloth.
12
34
5
13
13. LumenEye®X1 - Cleaning & Disinfection: Instructions
Verify that the handset is in working order
prior to cleaning.
6
12

IFU-101-1.0
Please repeat step 8 using the Tristel
Sporicidal Wipe.
Take a Tristel Sporicidal Wipe and unfold it
in the palm of your hand. Do not shake the
Activator Foam bottle. Dispense 2 full doses
of Tristel Activator Foam onto the Sporicidal
Wipe.
Remove a Tristel Pre-Clean Wipe from the
sachet and unfold it in the palm of your
hand. Fully wipe the device including
cable, starting from the handle to the tip
of the camera. Ensure all visible soiling
is removed. Pay particular attention to
indentations and ridges.
10 12
11
14
7 8
Thoroughly wipe the indentations in the
manifold port. Wrap the wipe around a
Q Tip and use this to reach every corner.
Squeeze liquid from the wipe onto the
device to ensure sucient coverage, if
required.
Manifold
port area
Fold the Sporicidal Wipe in on itself
(corners to middle) and scrunch for 15
seconds to generate chlorine dioxide.
Ensure that the wipe is completely
covered in foam, but do not squeeze
out the liquid.
910
Fully wipe the device including cable with
the activated Tristel Sporicidal Wipe starting
from the handle to the tip of the camera,
ensuring all surfaces are visibly wet and
sucient fluid has been passed from the
wipe to device. Pay particular attention to
indentations and ridges.
x 2
doses
Pre Clean
Wipe
Sporicidal
Wipe
A
c
t
i
v
a
t
o
r
F
o
a
m
Manifold
port area
10
Wrap
wipe
around
Q Tip
Wrap
wipe
around
Q Tip

IFU-101-1.014
Perform a visual inspection of the device
and verify it is clean following use and
before storing away. If the device is not
visibly clean, repeat cleaning steps 1-14.
8
15
Rinse
Wipe
Fully wipe the device including cable with
the Tristel Rinse Wipe. Ensure all surfaces are
wetted once and foam and other residues
from the previous steps are removed.
Place the LumenEye®X1 into the docking
case for storage (see section 9) ensuring
the docking case is also clean, disinfected
and maintained according to the
procedures detailed in section 10.
Use a lint-free soft dry cloth to thoroughly
dry the LumenEye®X1.
14
16
13
15
14. Charging Instructions
Caution: The device should
only be charged if it is not in
use (i.e. not connected to a
patient)
Caution: Any charger used
to charge the tablet must
comply with the requirements
of the IEC 60601-1
The LumenEye®X1 device does not require any charging and is powered by the tablet.
Note: The tablet used with the LumenEye®X1, must be charged following the
manufacturer’s instructions.
IFU-101-1.0
15. Software Compatibility
The LumenEye®X1 is intended to be used with the CHiP software which has
been developed by SurgEase. The LumenEye®X1 can be used with other
commercially available camera-feed processing software. Using alternative
software is done so at the user’s own risk.
16. Warranty
SurgEase warrants LumenEye®X1, when new, to be free from defects
in material and workmanship and to perform in accordance with the
manufacturer’s specifications for a period of one year from the date of first
use when purchased from SurgEase or any of the authorised distributors
or agents. After this period, it should be sent back to the manufacturer for
servicing. SurgEase will either repair or replace any components found to be
defective or at variance from the manufacturer’s specifications within this time
at no cost to the customer. It shall be the purchaser’s responsibility to return
the LumenEye®X1 to SurgEase or an authorised distributor, agent, or service
representative. This warranty does not include breakage or failure due to
tampering, misuse, neglect, accidents, modification, or shipping. This warranty
is also void if the instrument is not used in accordance with the manufacturer’s
recommendations or if it is repaired by any agent other than SurgEase or an
authorised agent. First-use date determines warranty requirements. No other
express warranty is given. Further technical information is available from the
manufacturer.
17. Life of Product & Servicing
All single-use consumables have a shelf-life of 2 years.
The expected service life of the LumenEye®X1 system is 1 year.
Under no circumstances should you attempt to repair or service the device
yourself. Inspection and repair should only be performed by SurgEase or an
authorised agent.
If your device requires repair, please contact us on: +44 (0)1282 690090
16

IFU-101-1.0 17
16
18. Disposal
This product is required to comply with the European Union’s Waste Electrical
and Electronic Equipment (WEEE) Directive 2002/96/EC. It is marked with the
following symbol:
This symbol indicates that the product should not be disposed of in household
waste, according to the WEEE Directive (2002/96/EC) and State Law. This
product should be returned to the manufacturer when the warranty expires.
Improper handling of waste may negatively impact the environment and
human health. Full co-operation with appropriate disposal advice improves the
use of natural resources.
For more information about recycling this product, contact SurgEase.

IFU-101-1.0
19. Troubleshooting
18
Condition Description and Corrective Action
Interrupted Feed If the video feed is lost or frozen, ensure that
the USB connector is securely in the correct
port. If this issue continues, ensure that there
are no other electronic devices in proximity
that could cause interference.
Unclear Feed If the video is foggy or unclear ensure that
the camera is clean. (wipe it according to
Section 13.0, page 13)
LumenEye® X1 not recognised Ensure that the cable is fully inserted in the
dedicated port on the tablet.
Manifold removal diculty Press down on the button of the manifold
and slide away from the endoscope. Make
sure the button is pressed firmly with no
interference (as seen in Section 11.10, page
11)
Video feed dark If you notice any LED failure around the
camera, please contact our technical
support team.
Lack of air pressure Ensure the Manifold is properly engaged
with both the Sheath and endoscope.
Follow instructions in Section 11, page 10.
Cable damage If you notice any damage on the cable, do
not use and contact the manufacturer.
Manifold or Sheath
engagement issues
Ensure the manifold and sheath have been
mounted on the device in the correct
orientation using the arrows as visual cues.
Use instructions in Section 11, page 10.
Obturator removal diculty Use instructions in Section 11, page 10.
For any enquiries, please contact technical support on: +44 (0) 330 043 6989
For any troubleshooting associated with the tablet, please refer to
manufacturer’s instructions.
Table of contents