Thor Medical Systems SpiroTube User manual

SpiroTube
WaveFront™PC Spirometer
User Manual
english
thormed_eu_eng r59
2012-10-12
web: http://www.thormed.com
e-mail: [email protected]
tel: +36 20 5837564
fax: +36 1 2093082


CONTENTS
Contents
1 Introduction ......................... 4
1.1 Intended use . . . . . . . . . . . . . . . . . . . . . . 4
2 Important safety warnings ................. 6
2.1 Danger of cross-contamination . . . . . . . . . . . . . 7
2.2 The Flowmeter . . . . . . . . . . . . . . . . . . . . . 7
2.3 The bacterial filter . . . . . . . . . . . . . . . . . . . . 7
2.4 Unforeseen errors . . . . . . . . . . . . . . . . . . . . 8
3 Description of the instrument ............... 9
3.1 General description . . . . . . . . . . . . . . . . . . . 9
3.2 Technical specification . . . . . . . . . . . . . . . . . 9
3.3 Labels and symbols . . . . . . . . . . . . . . . . . . . 11
4 Operation of SpiroTube .................. 13
4.1 Operation using USB connection . . . . . . . . . . . . 13
5 Maintenance ........................ 16
5.1 Disinfecting the tube . . . . . . . . . . . . . . . . . . 16
6 Declaration of EC conformity ............... 20
7 Limited Warranty Conditions ............... 21
3

1 INTRODUCTION
1 Introduction
1.1 Intended use
User Category
The spirometer measures a series of parameters relating to human
respiratory function. The product is therefore intended for use by a
doctor or by a nurse practitioner under the supervision of a doctor.
Qualification and experience required
The correct use of the instrument, the interpretation of the test
results plus the maintenance of the instrument,and in particular the
avoidance of cross-infection, all requires qualified personnel.
Operating environment
The operation of the instrument is foreseen within a doctor’s office or
within a hospital.
The instrument is not intended for use in an operating theatre or in the
presence of inflammable liquids or detergents, nor in the presence of
inflammable anesthetic gases or oxygen or nitrogen gases.
The instrument is not designed to be used in direct air currents (e.g.
wind), sources of heat or cold, direct sun rays or other sources or light
or energy, dust, sand or any other chemical substances.
The user is responsible to check the suitability of the ambient
conditions both for the storage and for the use of the instrument.
Patient effect on the use of the instrument
A spirometry test should only be carried out when the patient is at
rest and seated in a suitable condition for the test. A spirometry
test requires the collaboration of the patient; the patient must make a
complete forced expiration in order to have a meaningful test result.
4

1 INTRODUCTION
Limitations of use - Contraindications
An analysis of the results of a spirometry test is not in itself sufficient to
make a correct diagnosis of the patient’s clinical condition. A detailed
clinical history of the patient is also required together with any other
tests suggested by a doctor.
Test comments, a test interpretation and suggested courses of
treatment must be given by a doctor.
Any symptoms that the patient has at the time of the test must be
carefully considered before a spirometry test is made. The user is
responsible to assess both the mental and the physical capacity of
the patient to make a correct test and the user must also assess the
degree of collaboration for each test carried out.
Special attention should be given to testing elderly patients, children
and handicapped people. The instrument should never be used
when it is possible or probable that the validity of the results may be
compromised due to any such external factors.
5

2 IMPORTANT SAFETY WARNINGS
2 Important safety warnings
The safety and the correct performance of the instrument is warranted
only when the warnings and the safety rules are correctly observed.
The manufacturer accepts no responsibility for problems or damage
caused by the failure of the user to follow these instructions correctly.
The instrument must be used as described in the Users Manual
with particular attention to section 1.1 Intended use and only original
spares and accessories as specified by the manufacturer may be
used.
The maintenance operations detailed in this manual must be carried
out precisely. If these instructions are not followed this can cause
measurement errors and/or an incorrect interpretation of measured
values.
Any modifications, adjustments, repairs or reconfiguration must be
made by the manufacturer or by a qualified person authorized by the
manufacturer. Never attempt to make a repair oneself.
High-frequency emissions may interfere with the correct operation of
the instrument. For this reason, certain minimum clearances (a few
meters) should be observed when high-frequency appliances such as
a TV, radio, portable phone etc and other electronic units are operated
at the same time in the same room.
If the instrument is connected to any other instrument, then in order to
maintain the essential safety characteristics according to IEC 60601-1
only equipment which complies to the current safety regulations may
be used.
For the recycling of the spirometer, accessories, plastic consumable
materials (bacterial filter), use only the appropriate containers or
better return all such parts to the seller of the instrument or to a
recycling centre. All appropriate local regulations must be followed.
6

2 IMPORTANT SAFETY WARNINGS
2.1 Danger of cross-contamination
A disposable bacterial filter is required to connect a patient to the
spirometer to avoid cross-contamination. In order to avoid exposing
the patient to the critical danger of cross contamination before each
spirometry test a new monouse bacterial filter must be used for each
patient.
2.2 The Flowmeter
Do not allow dust or foreign bodies to enter the Flowmeter, to avoid
incorrect functioning and possible damage.
The presence of any impurities such as hairs, sputum, threads etc
within the body of the Flowmeter may seriously compromise the
accuracy of the measurements.
2.3 The bacterial filter
We suggest you to use bacterial filter for every measurement
preventing cross-contaminations. The bacterial filter should be
placed on the end of the tube so that it is between the Flowmeter
and the patient. The blue arrow on the device indicates the direction
of the expiratory air flow.
FlowMeter with bacterial filter (illustration)
Any monouse bacterial filter included with the instrument is supplied
only as a guide to the correct type and dimensions of the bacterial
filter required for this instrument, and they are clean but not sterile.
7

2 IMPORTANT SAFETY WARNINGS
To purchase appropriate bacterial filter we suggest that you contact
your local distributor who supplied the spirometer.
The use of a mouthpiece made from an inappropriate material could
modify the bio-compatibility and could be the cause of an incorrect
functioning of the instrument and of incorrect test results.
The user is responsible to obtain the correct type of bacterial filter
for the instrument. Those required are standard type with an outside
diameter of 30mm; they are commonly used and in general easily
procured.
2.4 Unforeseen errors
Errors in measurement or in interpretation can also be caused by:
• use by non-qualified or non-trained personnel, lacking ability or
experience
• user error
• use of the instrument outside the guidelines described in this
Users Manual
• use of the instrument even when some operational anomalies
may be encountered
• non-authorized servicing of the instrument
8

3 DESCRIPTION OF THE INSTRUMENT
3 Description of the instrument
SpiroTube is a simple to operate, precise pocket spirometer (the
sensor weight is only 150 grams) able to measure the most important
functional respiratory parameters with a quality control check on the
test carried out.
3.1 General description
The instrument has the following user friendly features:
• Plug-and-play operation
• Automatic internal calibration
• No moving parts
SpiroTube is intended for any doctor, from a family doctor to a
specialist, requiring a small and compact instrument able to make a
full spirometry test.
The sensor for flow and volume measurement is an Ultrasonic system
based on the WaveFront™ ultrasonic multiple-path principle. This
principle guarantees accuracy plus reproducibility of the measure-
ment.
3.2 Technical specification
Here follows a complete description of the instrument and of the flow
and volume measurement system.
Communication port/interface:
Connection to PC via USB or optional RS232
USB Connector type:
Standard 5-pin mini B type
Dimensions of the Device:
27x60x170 mm
Dimensions of the Flow tube:
⌀30 mm X 150 mm
9

3 DESCRIPTION OF THE INSTRUMENT
Weight:
145 grams
Flow/volume measurement system:
WaveFront™ technology
Measurement principle:
WaveFront™ ultrasonic multiple-path
Maximum volume:
± 20 L
Flow range:
± 18 L/s
Volume accuracy:
± 3% or 50 mL
Flow accuracy:
± 3% or 50 mL/s
Sample rate:
100 Hz
Dynamic resistance at 14 L/s:
< 110 Pa/L/s
Level of electrical protection:
BF
Protection against water ingress:
Standard instrument
Operating and storage conditions:
Temperature: 10-40°C
Relative humidity: 5 - 95% without condensation
10

3 DESCRIPTION OF THE INSTRUMENT
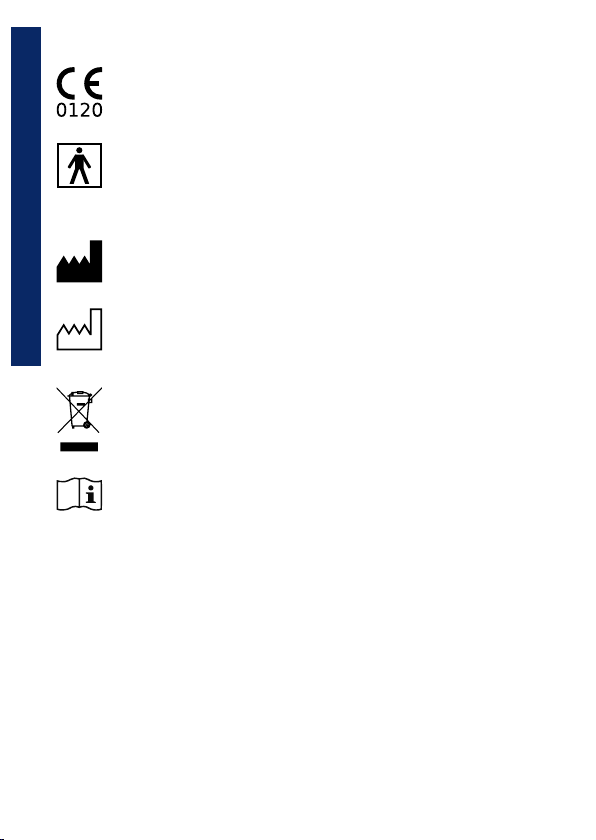
3.3 Labels and symbols
Product identification label
The identification label on the backside of the housing shows the
product name, and additionally the following:
• Manufacturer’s name and address
• Product conformity marking, in line with the CE 93/42 guidelines
• Serial number of the device
• Web site of the manufacturer
11
3 DESCRIPTION OF THE INSTRUMENT
Description of symbols used on the label
CE mark for medical devices. The product is conform to the
requirements of the 93/42/CEE medical devices directive.
Electrical safety symbol. In accordance with the EN 60601-
1 the product and its component parts are of type BF and
therefore protected against the dangers of direct and indirect
contact with electricity.
Symbol for ”Manufacturer.” This symbol is adjacent to the
name and address of the manufacturer.
Symbol indicating the ”date of manufacture.” The symbol
is adjacent to the date that the product was manufactured,
expressed as four digits for the year.
Symbol indicating ”Not for general waste.” This symbol marks
devices that are reusable and not contaminated at the end of
the device life.
Symbol for ”Caution, consult accompanying documents” and
”Attention, see instructions for use.”
12

4 OPERATION OF SPIROTUBE
4 Operation of SpiroTube
SpiroTube is a sensor device, which can be connected to laptops and
PCs via USB (cable) connection.
Please read installation instructions below for the proper work-
ing.
4.1 Operation using USB connection
SpiroTube drivers are installed together with ThorSoft Spirometry
Software. Please prepare your SpiroTube device and the shipped
USB cable for the installation.
IMPORTANT WARNING! You must start ThorSoft Spirometry
Software installer before you connect first the SpiroTube device.
The installer sets up the system to be ready to accept the
SpiroTube device. Please follow carefully the instructions of the
installer and connect the device as described below when it is
necessary.
There are fixed USB cable and mini usb connector equipped versions
of SpiroTube. The fixed USB cable equipped device has a persistent
USB cable, which cannot be detached from the sensor.
Fixed USB cable equipped sensor
13

4 OPERATION OF SPIROTUBE
The mini USB connector equipped device is shipped with a detach-
able USB cable. Please connect the smaller end of the USB cable to
the SpiroTube device taking care of the orientation of the plug.
The USB connector location on the device
The connected SpiroTube device
For connecting the USB cable to your PC or laptop, please find the
USB connector. It is typically located on the back of the desktop PCs
or on the side of laptops. A USB symbol shall be somewhere close
to the connector.
The USB symbol
14

4 OPERATION OF SPIROTUBE
USB connector on the back of the PC
Please connect the bigger end of the cable to the PC watching the
proper orientation of the plug.
Connecting the USB cable to laptop
15

5 MAINTENANCE
5 Maintenance
The Flowmeter used by SpiroTube guarantees the maximum mea-
surement accuracy and has the great advantage of not requiring ev-
eryday calibration. To ensure the maximum accuracy of the respira-
tory sensor, it is recommended to make a simple cleaning operation
in case of extensive use. It is a good practice from time to time to
make a visual check inside the tube to ensure that no hairs, dust or
foreign bodies of any kind have collected within the tube. Such an
occurrence could undermine the accuracy of the measurements.
SpiroTube is an instrument which requires very little maintenance.
The only regular maintenance operations required are:
• Cleaning and checking of the flow meter.
ATTENTION
• In order to understand the proper disinfection process
please observe section 5.1 Disinfecting the tube.
5.1 Disinfecting the tube
The disinfection process was tested and validated using INSTRUMED
as disinfection liquid. If you intend to use disinfection liquid other
than INSTRUMED please consult your local sales representative. IN-
STRUMED is a cleansing instrument disinfectant concentrate which
uses the latest in active agents, adjuvants and corrosion protection
compounds, with a wide anti-microbial spectrum of application. IN-
STRUMED is a yellow colored, mildly viscous product with a distinc-
tive aroma, which allows it to be distinguished from other medical in-
strument disinfectants.
Preparation of the disinfectant solution
Using an appropriately large container, fill with 10 liters of tap water at
a temperature not warmer than 40 °C. To this add the disinfectant to
the appropriate cubic volume, for example in the case of a 2% solution
16

5 MAINTENANCE
add 2dl, for a 1% solution add 1dl, and so on.
The working solution must always be prepared fresh before being
used.
Appropriate concentrations and exposure time
• 3% solution effective within 15 minutes
• 2% solution effective within 30 minutes
• 1% solution effective within 60 minutes
In the solution sterilization occurs with
• 5% solution effective within 3 hours
Disinfection steps
Step 1: Prepare 1%, 2% or 3% solution from the INSTRUMED as
described above
Step 2: Cover hermetically one of the end of the flowtube with the
shipped cup.
Step 3: Pour the prepared solution in the tube to leaving space only
for covering the other side the tube
17

5 MAINTENANCE
Pouring the solution in the tube
Step 4: Leave the solution in the tube for the specified time described
above
Step 5: Remove the upper cup and pour the solution out of the tube
Step 6: After flushing of the fluid carefully wipe the outer perimeter
of both ends of the flowtube with the disinfectant solution to
prevent the patient from cross infection
Wiping the outer perimeters with disinfectant
18

5 MAINTENANCE
Step 7: Flush the tube with plenty of distilled water
Step 8: Wait for the tube to dry or dry the tube with a ventilator.
IMPORTANT WARNINGS
• Only the flowtube can be disinfected. Never put the device
itself under a running tap (or other liquid) as irreparable
damage may be caused.
• If you intend to use disinfection liquid other than IN-
STRUMED please consult your local sales representative.
ATTENTIONS using INSTRUMED
• It is forbidden to mix with other cleansers or disinfectants!
• R22: Harmful if swallowed
• R34: Causes burns
• S2: Keep out of the reach of children
• S13: Keep away from food, drink and animal feeding stuffs
• S25: Avoid contact with eyes
• S26: In case of contact with eyes, rinse immediately with
plenty of water and seek medical advice
• S28: After contact with skin, wash immediately with plenty
of water
• S36/37/39: Wear suitable protective clothing, gloves, gog-
gles and facemasks
• S45: In case of accident or if you feel unwell seek medical
advice immediately (show the label where possible)
19

6 DECLARATION OF EC CONFORMITY
6 Declaration of EC conformity
Manufacturer
Thormed Kft.
Bogdánfy u. 10/a., Budapest, 1117, Hungary
Product
Spirometer
Model number
SpiroTube
Classification
Class IIa
Council Directive 93/42/EEC of MDD,
Annex IX, rule 10
Declaration
We hereby declare that the above listed products comply to
the provisions of the Council Directive 93/42/EEC for medical
devices. All supporting documentation is retained under the
premises of the manufacturer.
Applied standards
IEC 60601-1:2005 EN 980:2008
IEC 60601-1-2:2007 EN 1041:2008
ISO 26782:2009 ISO 14971:2007
Notified Body
SGS United Kingdom Ltd.
Systems & Services Certification;
202B World Parkway Weston super Mare, BS22 6WA UK
EC Certificates
Directive 93/42/EEC HU09/6306
ISO 13485:2003 HU09/6307
ISO 9001:2008 HU09/6308
0120
20
Table of contents