Thoratec HEARTMATE ICU COVER User manual

Thoratec Corporation
HEARTMATE®ICU COVER
INSTRUCTIONS FOR
U
SE
Thoratec Corporation continually strives to provide the highest quality of products for mechanical
circulatory support. Specifications may change without notice. HeartMate, Thoratec, and the
Thoratec logo are registered trademarks, and HeartMate 3 is a trademark of Thoratec
Corporation.
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
©2017 Thoratec Corporation.
Document: 10004064.B Publication Date: 10/2017


HeartMate ICU Cover
Instructions for Use 1
Section I: Preface
This manual contains information needed to properly and safely use
the HeartMate® ICU Cover. New users should read this document in
its entirety before system operation. For experienced practitioners, this
manual may serve as a reference.
As with all prescription medical devices, clinical procedures should be
conducted under the direction of the prescribing physician. The
professional staff at Thoratec regularly provides laboratory training
and on site, in-service programs. Additional training materials are
available for independent learning.
For assistance in setting up, using, or maintaining the HeartMate ICU
Cover or to report any unexpected operation, contact
Thoratec Corporation®. See Thoratec contact information on back
cover.
Section II: General Information
1.0 Description
The disposable, non-sterile, single-patient use ICU Cover is for
post-operative hospital use, for example in the ICU. The ICU Cover:
• Is a location management accessory to secure the System Controller
to a visible location (such as the patient’s gown) using the provided
Clip.
• This product is designed for use with the HeartMate 3™ System
Controller.
• Helps limit direct skin contact between the patient and the
System Controller.
• Provides a protective cover between any local contaminate and the
System Controller.

HeartMate ICU Cover
2Instructions for Use
Figure 1 displays the System Controller in the ICU Cover showing the
Clip and Usage Tag.
Clip
Usage Tag
Figure 1: System Controller in ICU Cover: Front View
Figure 2 displays the back view of the System Controller in the
ICU Cover showing the Strap and Clip.
Strap
Clip
Figure 2: System Controller in ICU Cover: Back View

HeartMate ICU Cover
Instructions for Use 3
The ICU Cover has a usage tag with numbers to help track the number
of days the ICU Cover has been used. One number should be marked
each day the ICU Cover is used; if desired, write the date on the usage
tag. The ICU Cover should be replaced after all seven numbers have
been marked, or if it becomes soiled or damaged.
2.0 Indications for Use
The ICU Cover is indicated for hospital use by post-operative patients,
for example in an ICU setting. This product is only intended for use
when the patient is confined to bed.
For the HeartMate 3 Left Ventricular System Indications for Use, please
refer to the HeartMate 3 Instructions for Use.
3.0 Contraindications
There are no known contraindications for the use of the ICU Cover.

HeartMate ICU Cover
4Instructions for Use
4.0 Warnings
WARNING !
• This product is designed for single patient use only, and
should be disposed of after 7 days, or earlier if it becomes
soiled or damaged and a replacement cover is needed.
• This product is designed for use with the HeartMate 3
System Controller.
• Inappropriate placement of the ICU Cover with the
HeartMate 3 System Controller can cause serious injury or
burns. Do not place in prolonged direct patient skin contact.
Do not place it underneath the patient or under a blanket.
• Do not use when the patient is ambulatory or at home.
• No modification of the ICU Cover is allowed. Do not use the
ICU Cover with any other accessories.
• Do not attempt to clean the ICU Cover. Replace it with a new
unit if the ICU Cover becomes soiled.
• Do not place in a location that can have prolonged contact
on bare skin or can be covered by the body or insulating
material, such as a blanket.
• Product and Medical Waste Disposal
Dispose of the damaged or used ICU Cover according to
applicable local, state, and federal regulations. For
additional product disposal information, contact Thoratec
Corporation for assistance.

HeartMate ICU Cover
Instructions for Use 5
5.0 Cautions
6.0 Adverse Events
There are no known or anticipated adverse events associated with the
use of the ICU Cover.
CAUTION !
• Do not attach any other objects or items to the ICU Cover.
• Ensure that the System Controller with the ICU Cover is
securely attached to a visible location (such as the patient’s
gown). Ensure that it is not in prolonged direct patient skin
contact or placed underneath the patient.
• When installing or removing the ICU Cover, avoid pulling on
or moving the driveline at the exit site. Pulling on or moving
the driveline can keep the exit site from healing or damage
an already healed exit site. Exit site trauma or tissue damage
can increase the patient’s risk of getting a serious infection.
• When installing or removing the ICU Cover, do not drop the
System Controller or subject it to extreme physical shock.
When installing or removing the ICU Cover, do not twist,
kink, or sharply bend the driveline, System Controller power
cables, Power Module patient cable, or Mobile Power Unit
patient cable.
• With the ICU Cover installed, the driveline safety tab on the
System Controller is covered. To access the driveline safety
tab on the System Controller for driveline connection, it is
required to remove the ICU Cover.

HeartMate ICU Cover
6Instructions for Use
Section III: Instructions
1.0 Installation and Marking of Usage Tag
1. Insert the System Controller into the ICU Cover immediately
after the Backup Battery is installed or the patient is moved
into ICU.
2. Ensure that the System Controller display is visible
(Figure 1).
Figure 1: System Controller in ICU Cover

HeartMate ICU Cover
Instructions for Use 7
3. Close the ICU Cover with the VELCRO®strap located on the
top of the ICU Cover to hold the System Controller in place
(Figure 2).
VELCRO®Strap
Figure 2: Close the VELCRO®Strap of the ICU Cover
4. If needed, re-thread the strap on the back of the ICU Cover
(depending on the orientation to clip the System Controller)
and snap the button to close it (Figure 3).
Clip
Strap
Figure 3: ICU Cover: Back View

HeartMate ICU Cover
8Instructions for Use
5. Clip the System Controller with the ICU Cover installed to a
secure visible location (such as the patient’s gown), and
ensure that it does not come in direct skin contact or pull on
the Driveline.
Complete the following steps to clip the ICU Cover:
a. Open the Clip by flipping the lever arm (Figure 4).
Figure 4: Opening the Clip
b. Slide the fabric into the Clip opening (Figure 5).
Figure 5: Sliding Fabric Into the Clip Opening

HeartMate ICU Cover
Instructions for Use 9
c. Press down on the Clip lever arm until it closes into place
(Figure 6).
d. Gently tug to ensure that the System Controller with the
ICU Cover is securely attached.
Figure 6: Securing the Clip
6. Ensure that the System Controller with the ICU Cover
installed:
• Is not kept covered by an insulating material, such as a
blanket.
• Will not come in prolonged direct contact with the
patient’s skin.
7. On day one of using the ICU Cover, mark the first number
on the ICU Cover Usage Tag (Figure 7); if desired, write the
date.
Figure 7: Unused Usage Tag
8. Mark one number daily.

HeartMate ICU Cover
10 Instructions for Use
9. The ICU Cover shall be replaced when all the numbers have
been marked.
2.0 Removal
1. Replace the ICU Cover when all the numbers on the Usage
Tag are marked, or when the ICU Cover is soiled or
damaged.
2. Remove the VELCRO®strap.
3. Remove the ICU Cover off of the System Controller by
pulling the Usage Tag while holding the System Controller
black and white power cables (Figure 8).
Avoid pulling the Driveline.
Figure 8: Removing the ICU Cover
Power Cables
Usage Tag
Driveline

HeartMate ICU Cover
Instructions for Use 11
HeartMate ICU Cover
12 Instructions for Use
Section IV: Symbols
This section describes the symbols that are used on the Thoratec components,
accessories, or packaging.
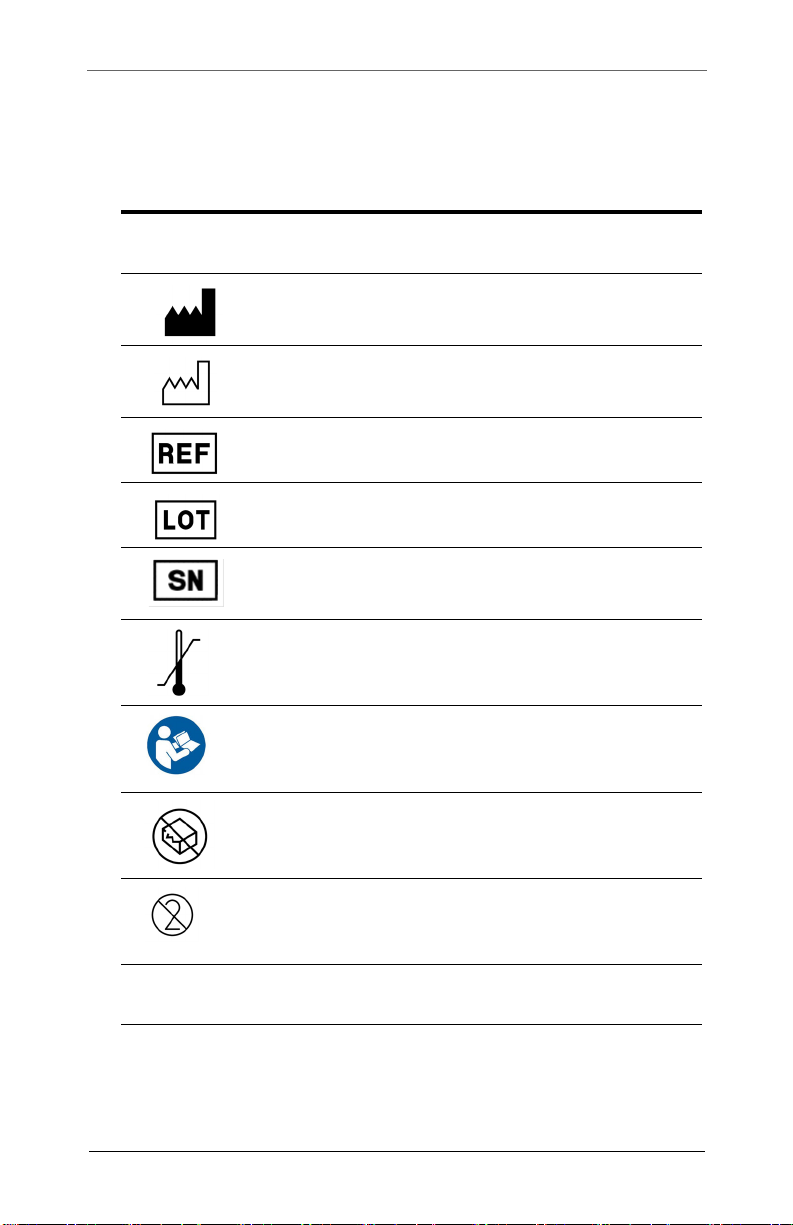
SYMBOL DESCRIPTION
Manufacturer
Date of manufacture
Catalogue number
Batch Code
Serial number
Temperature Limitation
Follow Instructions for Use
Do not use if package is damaged
Do not reuse
1 Quantity of contents

HeartMate ICU Cover
Instructions for Use 13
Section V: Acceptable Operating
Conditions
For safe and optimal use of Thoratec system components, follow the operating
guidelines listed here. Operating system components outside of the
environmental parameters listed below may affect device operation or result in
equipment failure.
TYPE DETAILS
Acceptable Temperature Range -4°F to 131°F
(-20°C to 55°C)
Air Pressure
mm Hg (hPA) Not applicable

HeartMate ICU Cover
14 Instructions for Use


Manufactured by:
Thoratec Corporation
6035 Stoneridge Drive
Pleasanton, CA 94588
USA
Tel: 925-847-8600
Fax: 925-847-8574
Internet: www.thoratec.com
Thoratec Corporation 6035 Stoneridge Drive Pleasanton, CA 94588 Tel: 925-847-8600
Toll Free: 800-528-2577 Fax: 925-847-8574 www.thoratec.com. © 2017 Thoratec Corporation.
Table of contents