Trividia TRUEresult User manual

INTRODUCTION
Welcome to the TRUEresult Blood Glucose
Monitoring System
Our Commitment to You
TRUEresult is a simple, accurate way to test whole
blood glucose (sugar) level, anytime, anywhere.
Our goal is to provide quality healthcare products
and dedicated customer service. For questions
about TRUEresult products, please see cover for
phone number.
is booklet contains all the information needed
to get the most from the TRUEresult System. To
start testing quickly, please see the Quick Reference
Guide found inside the cover of this Owner’s
Booklet.
Please read complete Owner’s Booklet and
all product Instructions for Use before
using the System.
1
Use By
Date
For in vitro
Diagnostic
Testing Only
Lot
Number
Authorised
Representative
SYMBOLS:
Keep
Dry
Caution
Single
Use
Only
Attention! Read
Instructions
for Use.
Sterile
Control
Storage
Temperature
Range
Manufactured
By
Serial
Number
Control
Level
Biological
Risk
Do Not
Resterilise
For
Assistance
Call
Date of
Manufacture

2
IMPORTANCE OF BLOOD GLUCOSE MONITORING
e more you know about diabetes, the better
you will be able to take care of yourself. A Doctor
or Healthcare Professional will determine target
ranges for blood glucose results and how oen to
test. Having most results within the target ranges
shows how well a treatment plan is working to
control glucose levels. Keeping most results within
the target ranges helps slow or stop complications
of diabetes.
NEVER change a treatment plan without consulting
with a Doctor or Healthcare Professional.
Use of the TRUEresult Blood Glucose Monitoring
System in a manner not specied in this Owner’s
Booklet is not recommended and may aect your
ability to determine true blood glucose levels.
e TRUEresult System is an in vitro
(outside body) quantitative system that is used for
self-testing and point-of-care testing of only human
whole blood. e most accurate results come from
using fresh whole blood taken from the ngertip or
forearm (capillary) or from the vein (venous).

3
What you need to know when using the
TRUEresult System:
• Read all product instructions for use before
testing.
• Use only TRUEresult Test Strips and TRUEresult
Control Solution with TRUEresult Meter.
• To help prevent false high results, wash hands
before using the System to test blood, especially
aer handling fruit or other foods containing
sugar.
• Perform Control Tests before performing a blood
glucose test for the rst time (see Control Test).
• Remove only one Test Strip at a time from vial
when testing. Recap vial immediately aer re-
moving Test Strip.
• NEVER reuse Test Strips. NEVER wipe Test
Strips with water, alcohol or any cleaner. DO
NOT attempt to clean and re-use Test Strips.
Reuse of Test Strips will cause inaccurate results.
• NEVER add a second drop of sample to Test
Strip. Adding more sample to the Test Strip gives
an error message.
• Venous whole blood collected into sodium or
lithium heparin blood collection tubes may be
used for testing by Healthcare Professionals.
Use of EDTA blood collection tubes is not
recommended and may cause low results. Mix
tube contents gently before using.

4
IMPORTANT HEALTH and SAFETY
INFORMATION:
e TRUEresult Blood Glucose Monitoring System
is for one person use ONLY. DO NOT share
your Meter or your Lancing Device with anyone,
including family members. DO NOT use on more
than one person. ALL parts of the TRUEresult Blood
Glucose Monitoring System could carry blood-
borne pathogens aer use, even aer cleaning and
disinfection.
2,3
For cleaning and disinfecting the Meter, see Care,
Cleaning/Disinfecting. For cleaning and disinfecting
the lancing device, see the lancing device’s
Instructions for Use.
We suggest cleaning the Meter when visibly dirty
or if blood is on the Meter. Wash your hands
thoroughly with soap and warm water aer
handling the Meter, lancing device, or Test Strips
as contact with blood presents an infection risk.
Reuse of devices labeled for single-use may result
in product contamination and patient infection.

5
•
DO NOT perform capillary blood glucose
testing on critically ill patients. Capillary blood
glucose levels in critically ill patients with reduced
peripheral blood ow may not reect the true
physiological state. Reduced peripheral blood ow
may result from the following conditions (for
example):
4
~ shock
~ severe hypotension
~ severe dehydration
~ hyperglycaemia with hyperosmolarity,
with or without ketosis.
• Do not use TRUEresult for the diagnosis of
diabetes or for testing blood glucose in neonates
(newborns).
•
Do not use TRUEresult System during a xylose
absorption test. is may falsely raise glucose
results.
5
Please check with your Doctor before
using TRUEresult.

6
TABLE OF CONTENTS
Phone Number, Fast Test Guide,
Expected Results........................................... see covers
Introduction and Important Information ...........1
Know the System ..................................................8
Meter .......................................................................8
Test Strip...............................................................11
Control Solution ..................................................13
Getting Started ....................................................14
Quality Control Testing......................................15
Automatic Self-Test.............................................15
Control Test .........................................................16
How to Test Control Solution ...........................17
Testing Blood ......................................................20
Obtaining a Blood Sample .................................20
How to Test Blood Glucose ...............................22
Unusual Blood Glucose Test Results................25
Ketone Test Alert.................................................26
TRUEresult System and Laboratory Testing...27
Memory................................................................28
View Averages (7-, 14-, and 30-day) ................28
View Results .........................................................29

7
Meter Set Up........................................................30
Set Ketone Test Alert ..........................................32
Set Testing Reminders ........................................32
Care, Cleaning/Disinfecting ...............................34
Meter Care............................................................34
Control Solution Care.........................................35
Test Strip Care .....................................................35
Changing Battery.................................................36
Troubleshooting..................................................37
Messages................................................................38
Technical Information........................................40
Performance Characteristics..............................40
System Specications ..........................................44
Operating Range..................................................44
Chemical Composition.......................................44
EMC Safety Information ....................................45
References............................................................46
Notes....................................................................47

8
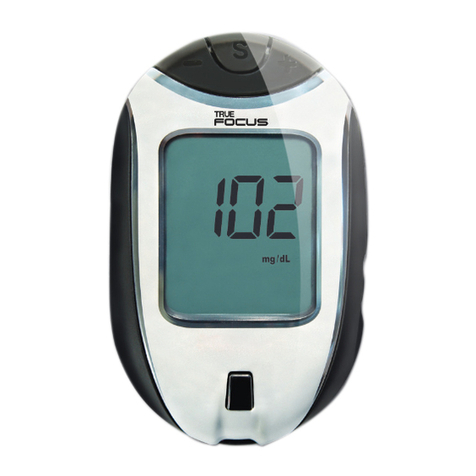
KNOW THE SYSTEM
Meter
“ + ” Button
Increase numbers in Time/Date Set Up for Meter
and Testing Reminders; turn on Testing Reminders
and Ketone Test Alert; add ALT Symbol; move
forward by date/time when viewing results in
Memory.
“ S ” Button
Turn Meter on to view Average values, to view
results in Memory, and to access Meter Set Up.
“ - ” Button
Decrease numbers in Time/Date Set Up for Meter
and Testing Reminders; turn o Testing Reminders
and Ketone Test Alert; remove ALT Symbol; move
backward by date/time when viewing results in
Memory.
Top of Meter
1 32

4
5
Back of Meter
6
Display Screen
Shows test results, messages, user prompts, other
information.
Test Port
Insert TRUEresult Test Strip here.
Strip Release Button
Releases Test Strip aer testing.
Battery Door
Use one non-rechargeable 3V lithium battery
(#CR2032), positive (“+”) side up (see Changing
Battery).
Meter Label
Contains serial number of Meter. Identies Meter
when calling for assistance.
Docking Station Contacts
Contacts used for uploading Meter results to a
computer using a Docking Station (Please call for
availability). 9
2
1
Front of Meter
3

10
Meter Display
Full Display Screen
1. Memory Symbol
2. Time, Date
3. Time is AM/PM
(Note: Not seen if factory set to 24 hour clock.)
4.
Result is from 7-, 14-, and 30-day Average
5. Day of Week
6. Test Result
7. Unit of Measure
(Note: Factory set, cannot be changed by user.)
8. Ketone Test Alert Symbol
9.
Testing Reminder Symbol
10. Temperature Symbol
11.
Drop Symbol - Apply blood or Control Solution
12. Alternate Site (ALT) Symbol
13. Battery Symbol
14. Control Symbol
3
4
2
1
7
6
5
8
9
10
11
12
13
14

11
Contact End -
Insert into Test Port with blocks
(contacts) facing up
.
Sample Tip -
Touch to top of sample (fresh,
capillary or venous blood or Control Solution)
aer inserting Contact End into Meter.
Sample Placement
Correct Incorrect
•
Do not touch Sample Tip to drop of sample
unless Contact End is inserted into Meter.
• Do not apply blood or Control Solution to top
of Test Strip.
•Do not smear or scrape drop with Test Strip.
• Do not apply more sample to the Test Strip
aer testing begins.
• Do not insert Sample Tip with sample into
Meter for testing. May damage Meter.
Test Strip
12
Top of Test Strip

Lot Number ( ) - Used for identication
when calling for assistance.
Use By Dates ( ) - Write date rst opened on
vial label. Discard vial and unused Test Strips if
either 4 months aer rst opening or date printed
next to on vial label has passed, whichever
comes rst.
Use of Test Strips or Control Solution past
the Use By Dates may give incorrect test
results. Discard out-of-date products and test
with new products.
Control Test Range - Range of numbers in
which Control Test result must fall to assure the
System is working properly.
12
Test Strip Vial Label
1
2
2
3
May 30, 2014
1
2
2
3
May 30, 2014
or

13
Control Solution Bottle Label
Lot Number ( ) - Used for identication
when calling for assistance.
Use By Dates ( ) - Write date rst opened on
bottle label. Discard bottle if either 3 months
aer rst opening or date printed next to on
bottle label has passed, whichever comes rst.
Control Solution Level (1, 2 or 3) - Testing
at least 2 levels of Control Solution is
recommended. Call the number on the cover of
this Booklet for information on how to obtain
dierent levels of Control Solution.
12
3
May 30, 2014
Control Solution

GETTING STARTED
e TRUEresult Meter comes with pre-set time, date,
Ketone Test Alert and all Testing Reminders o. Before
using Meter for the rst time or aer battery is changed,
check to make sure all of the Meter pre-set functions are
correct. Update any setting as needed (see Meter Set Up).
e Meter turns on when a Test Strip is inserted into the
Test Port or when “ S ” Button is pressed and released (see
Memory and Meter Set Up). Meter turns o when the Test
Strip is released from the Meter, “ S ” Button is pressed and
held for 20 seconds, or aer 2 minutes of non-use.
Always check your supplies before using.
• Check Meter for damage (cracked Display, missing
buttons, etc.). If damage is seen, do not use Meter. Call
for assistance.
• Check Test Strip vial for damage (cracked vial, broken
vial, etc.). Discard damaged vial and all contents (Test
Strips) and use a new vial of Test Strips for testing.
•
Write date rst opened on Test Strip vial. Check Use By
Dates (written and printed) before using any Test Strips
from the vial. Do not use if 4months aer rst opening
(written date) or if printed Use By Date has passed.
• Check Control Solution bottle for any leaks or broken
cap. Discard bottle and open a new one for testing.
• Write date rst opened on Control Solution bottle
label. Check Use By Dates (written and printed) before
using. Do not use if 3 months aer rst opening
(written date) or if printed Use By Date has passed.
14

15
Quality Control Testing
To assure you are getting accurate and reliable results,
TRUEresult oers two kinds of quality control tests.
ese tests let you know that your TRUEresult System
is working properly and your testing technique is good.
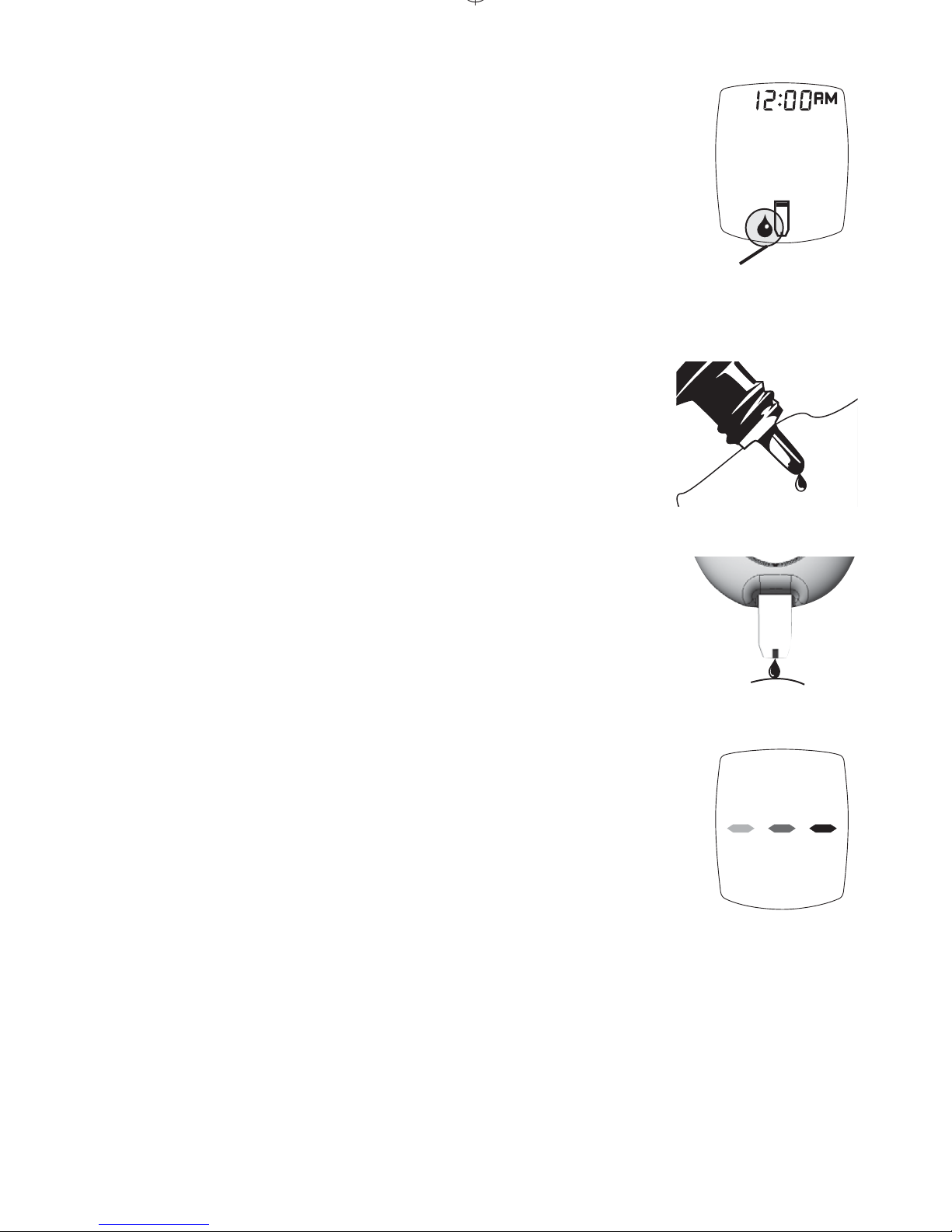
Automatic Self-Test
An Automatic Self-Test ensures that
the Meter is working correctly.
1. Wash hands and dry thoroughly.
2. Remove one Test Strip from the
Test Strip vial and insert Test Strip
into the Meter.
3.
Meter turns on. e full Display
appears and is replaced by the time
and the blinking Drop Symbol. e
Meter is working correctly and is ready
to perform a Control or blood test.
Do not use Meter if:
•
e full Display does not appear
(segments are missing),
•
e blinking Drop Symbol does not appear, or
•
An error message appears in the Display.
See Troubleshooting or call for assistance (see cover
of this Booklet for phone number).
Full Display
Contacts
Facing Up
16
Control Test
e Control Test checks that the System is working
correctly and testing technique is good. Use ONLY
TRUEresult Control Solution to perform Control
Tests. Perform Control Tests:
•
For practice before using the System for the rst
time,
•
When opening a new vial of Test Strips,
•
Occasionally as a vial of Test Strips is used,
•
If a Test Strip vial has been le opened or le in
extreme heat, cold, or humidity,
•
Whenever a check on performance of the System is
needed,
•
If results seem unusually high or low,
•
If Meter damage is suspected (Meter was dropped,
crushed, wet, etc.).
Performing Control Tests with more than one level
of Control Solution is recommended to ensure that
your System is working properly. ree levels of
TRUEresult Control Solution are available. Contact
place of purchase or use the number on the cover of
this Booklet for more information on how to obtain
dierent levels of Control Solution.
Ranges printed on Test Strip vial label are
for Control Test results only and are not
suggested levels for your blood glucose. Do
not drink Control Solution.

17
How to Test Control Solution
Use ONLY TRUEresult Control
Solution with the TRUEresult Meter.
1. Check supplies. See Getting Started.
2. Allow Control Solution, vial of Test
Strips and Meter to adjust to room
temperature (20°C-25°C).
Note: Running a Control Test at
temperatures outside the range
listed above may cause Control
Solution to read as a blood test.
3. Wash hands. Dry thoroughly.
4. Gently swirl or invert Control Solution bottle to
mix. DO NOT SHAKE!
5. Remove one Test Strip from vial. Close Test
Strip vial immediately. Use Test Strip quickly
aer removal from vial.
Note: If Test Strip has been out of the
vial too long before testing, an
error message appears upon
insertion of the Test Strip into the
Meter. Release and discard old
Test Strip. Use new Test Strip for
testing.
6. Insert Test Strip into Meter. Meter turns on
and shows blinking Drop Symbol and time. Do
not remove Test Strip.
Contacts
Facing Up
May 30, 2014
May 30, 2014
18
7. With cap removed, turn Control
Solution bottle upside down.
Gently squeeze one drop of Control
Solution onto a clean tissue. Wipe
o bottle tip with the tissue.
Note:
If Test Strip is removed before testing
is nished, an error message appears.
Release and discard old Test Strip.
Use new Test Strip for testing.
8.
Gently squeeze a drop of Control
Solution onto a small piece of
unused aluminum foil or clear
plastic wrap. Discard foil or plastic
wrap aer use.
Note:
Do not put drop on top of the Test
Strip.
9.
With Test Strip still in Meter, touch
Sample Tip to top of the drop of
Control Solution. Allow drop to be
drawn into Test Strip. Remove Test
Strip from drop when Meter beeps.
Dashes appear across the Display to
show Meter is testing.
Note: If Meter does not beep or begin testing soon
aer drawing up sample, release and discard
Test Strip. Repeat test with new Test Strip. If
problem persists, see Troubleshooting.
10. Aer testing is nished, the result appears in the
display with the Control Symbol.
Drop Symbol
Meter Testing
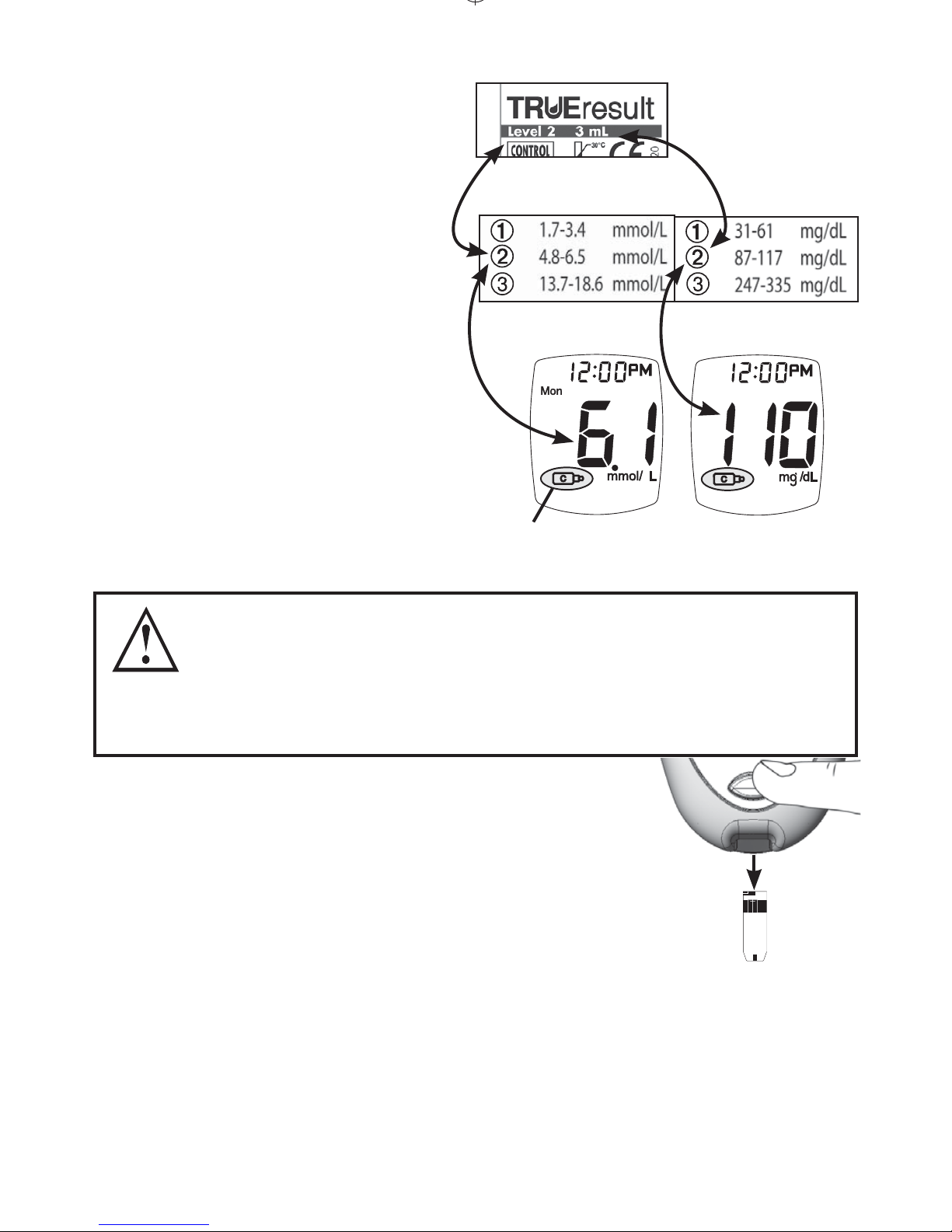
11.
Compare result to
Control Test Range
printed on Test Strip
vial label for Control
Solution Level you
are using.
If result is in range,
System can be used for
testing blood. If result
does not fall within range,
repeat test using a new
Test Strip.
If result is still outside range aer a second
Control Test, System should not be used for
testing blood. Call for assistance (see cover for
phone number).
12.
Aer result is shown, Strip Release
Button ashes. Hold Meter with Test
Strip pointing down. Press Strip Release
Button to release and discard Test Strip
in appropriate container. Meter turns o.
Recap Control Solution bottle tightly.
Note:
Removing Test Strip before result displays cancels
the test. An error message appears and the result
is not stored in Memory. Retest with a new Test
Strip and do not remove before result is displayed.
19
Control Symbol
Test Strip
Vial Label
Control Solution
Bottle Label

20
TESTING BLOOD
Obtaining a Blood Sample
Refer to lancing device’s Instructions for Use for
detailed instructions.
e lancing device is for single patient use
ONLY. For cleaning/disinfecting your lancing
device see Lancing Device Care in the lancing
device’s Instructions for Use. Wash your hands
thoroughly with soap and warm water aer
handling the Meter, lancing device, or Test Strips.
Contact with blood presents an infection risk.
•NEVER share lancets or lancing device.
• Lancets are for single use only. Do not reuse lancets.
• To help prevent false high results, wash hands
before using the System to test blood, especially aer
handling fruit or other foods containing sugar.
From Fingertip
1. Prepare ngertip by washing hands in warm, soapy
water. Rinse well. Dry thoroughly.
2. Place end of lancing device equipped with
lancet against tip of nger. Lance ngertip.
3. Set lancing device aside. To help blood drop form,
lower hand to waist level, gently massage nger from
palm to ngertip. Allow blood drop to form before
attempting to apply to Test Strip.
4. Recap and remove used lancet from lancing device.
Discard used lancet into appropriate container.
Note: Treat used Test Strips and lancets as a biological
risk. Dispose used Test Strips and lancets in
approved container.
Table of contents
Other Trividia Blood Glucose Meter manuals